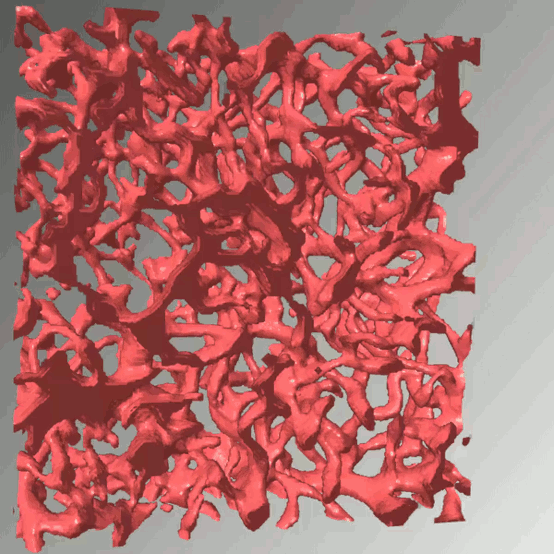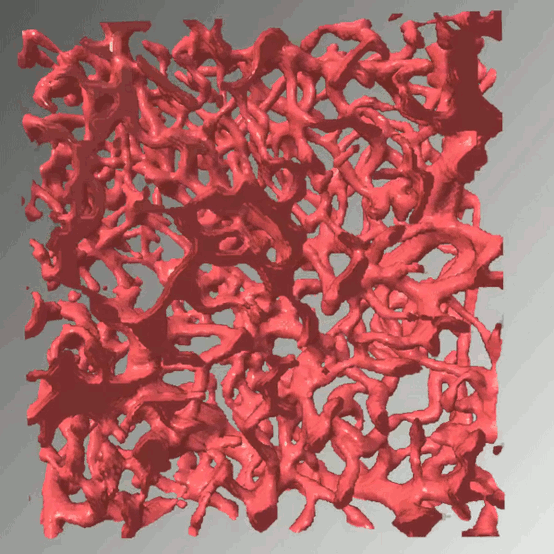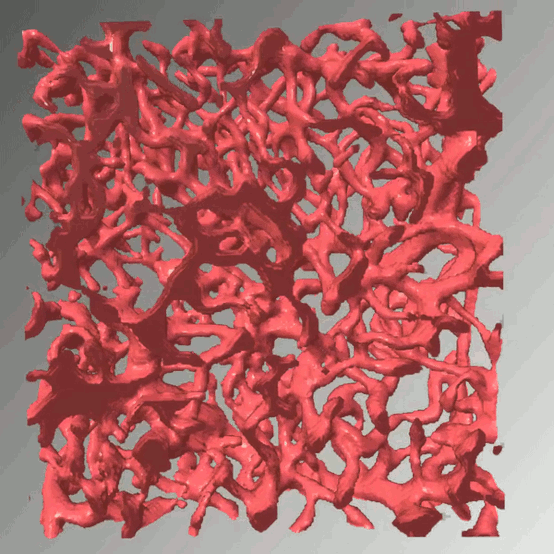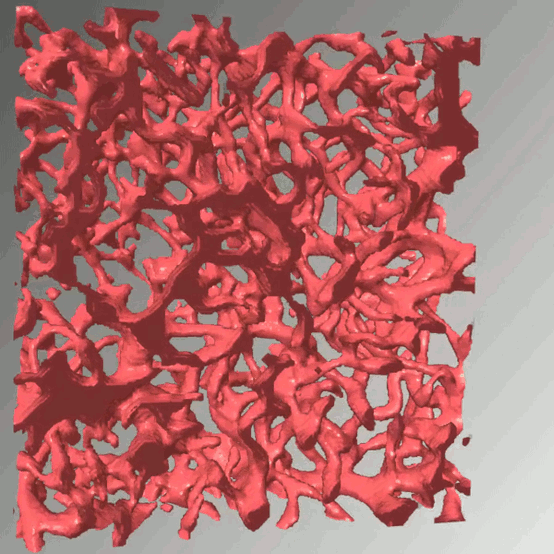
Access to molecular oxygen is essential for cell function and behaviour. Tools to measure this central parameter, in particular in vivo, are limited. We generate hypoxia reporter systems, which we use to understand the contribution of immune cells to acute and chronic hypoxia in cells, organs and entire organisms. For the automated analysis of the big data, which we derive with our reporter systems in large tissue volumes, we have developed dedicated deep learning pipelines. We extend and accompany the resulting massive imaging data by complementary annotation and supervision tools, which allows us to analyse hypoxia across scales. Our approach provides unprecedented insights into the dynamics and consequences of hypoxia and its connection to inflammation.
| Bauer N, Maisuls I, Pereira da Graca A, Reinhardt D, Erapaneedi R, Kirschnick N, Schäfers M, Grashoff C, Landfester K, Vestweber D, Strassert CA, Kiefer F. Genetically encoded dual fluorophore reporters for graded oxygen-sensing in light microscopy. Biosens Bioelectron 2023;221: 114917. Abstract
|
| Beckmann D, Kockwelp J, Gromoll J, Kiefer F, Risse B. SAM meets Gaze: Passive Eye Tracking for Prompt-based Instance Segmentation. NeuRIPS. 2023 Workshop on Gaze Meets ML 2023
|
| Haalck L, Mangan M, Wystrach A, Clement L, Webb B, Risse B. CATER: Combined Animal Tracking & Environment Reconstruction. Sci Adv 2023;9: eadg2094. Abstract
|
| Kockwelp J, Gromoll J, Wistuba J, Risse B. EyeGuide-From Gaze Data to Instance Segmentation. British Machine Vision Conference BMVC. 2023
|
| Kockwelp J, Thiele S, Kockwelp P, Bartsch J, Schliemann C, Angenendt L, Risse B. Cell Selection-based Data Reduction Pipeline for Whole Slide Image Analysis of Acute Myeloid Leukemia. 2022 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW) 2023: 1824-1833. Abstract |
| Bobe S, Beckmann D, Klump DM, Dierkes C, Kirschnick N, Redder E, Bauer N, Schäfers M, Erapaneedi R, Risse B, van de Pavert SA, Kiefer F. Volumetric imaging reveals VEGF-C-dependent formation of hepatic lymph vessels in mice. Front Cell Dev Biol 2022;10: 949896. Abstract
|
| Eliat F, Sohn R, Renner H, Kagermeier T, Volkery S, Brinkmann H, Kirschnick N, Kiefer F, Grabos M, Becker K, Bedzhov I, Schöler HR, Bruder JM. Tissue clearing may alter emission and absorption properties of common fluorophores. Sci Rep 2022;12: 5551. Abstract
|
| Peil J, Bock F, Kiefer F, Schmidt R, Heindl LM, Cursiefen C, Schlereth SL. New Therapeutic Approaches for Conjunctival Melanoma-What We Know So Far and Where Therapy Is Potentially Heading: Focus on Lymphatic Vessels and Dendritic Cells. Int J Mol Sci 2022;23Abstract
|
| Reimche I, Yu H, Ariantari NP, Liu Z, Merkens K, Rotfuß S, Peter K, Jungwirth U, Bauer N, Kiefer F, Neudörfl J-M, Schmalz H-G, Proksch P, Teusch N. Phenanthroindolizidine Alkaloids Isolated from Tylophora ovata as Potent Inhibitors of Inflammation, Spheroid Growth, and Invasion of Triple-Negative Breast Cancer. Int J Mol Sci 2022;23Abstract |
| Arasa J, Collado-Diaz V, Kritikos I, Medina-Sanchez JD, Friess MC, Sigmund EC, Schineis P, Hunter MC, Tacconi C, Paterson N, Nagasawa T, Kiefer F, Makinen T, Detmar M, Moser M, Lämmermann T, Halin C. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J Exp Med 2021;218Abstract
|
| Bian A, Jiang X, Berh D, Risse B. Resolving Colliding Larvae by Fitting ASM to Random Walker-Based Pre-Segmentations. IEEE/ACM Trans Comput Biol Bioinform 2021;18: 1184-1194. Abstract
|
| Huesmann K, Rodriguez LG, Linsen L, Risse B. The Impact of Activation Sparsity on Overfitting in Convolutional Neural Networks. Pattern Recognition. ICPR International Workshops and Challenges 2021: 130-145. Abstract
|
| Kirschnick N, Drees D, Redder E, Erapaneedi R, Pereira da Graca A, Schäfers M, Jiang X, Kiefer F. Rapid methods for the evaluation of fluorescent reporters in tissue clearing and the segmentation of large vascular structures. iScience 2021;24: 102650. Abstract
|
| Kong C, Bobe S, Pilger C, Lachetta M, Øie CI, Kirschnick N, Mönkemöller V, Hübner W, Förster C, Schüttpelz M, Kiefer F, Huser T, Schulte Am Esch J. Multiscale and Multimodal Optical Imaging of the Ultrastructure of Human Liver Biopsies. Front Physiol 2021;12: 637136. Abstract
|
| Redder E, Kirschnick N, Bobe S, Hägerling R, Hansmeier NR, Kiefer F. Vegfr3-tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities. PLoS One 2021;16: e0249256. Abstract
|
| Sadras T, Martin M, Kume K, Robinson ME, Saravanakumar S, Lenz G, Chen Z, Song JY, Siddiqi T, Oksa L, Knapp AM, Cutler J, Cosgun KN, Klemm L, Ecker V, Winchester J, Ghergus D, Soulas-Sprauel P, Kiefer F, Heisterkamp N, Pandey A, Ngo V, Wang L, Jumaa H, Buchner M, Ruland J, Chan W-C, Meffre E, Martin T, Müschen M. Developmental partitioning of SYK and ZAP70 prevents autoimmunity and cancer. Mol Cell 2021;81: 2094-2111.e9. Abstract |
| Hägerling R, Hoppe E, Dierkes C, Stehling M, Makinen T, Butz S, Vestweber D, Kiefer F. Distinct roles of VE‐cadherin for development and maintenance of specific lymph vessel beds. EMBO J 2018: e98271. Abstract
|
| Klemm S, Jiang X, Risse B. Deep distance transform to segment visually indistinguishable merged objects. In: Proc. of 40th German Conference on Pattern Recognition (GCPR), Stuttgart 2018: 422-433.
|
| Orlich M, Kiefer F. A qualitative comparison of ten tissue clearing techniques. Histol Histopathol 2018;33: 181-199. Abstract |