Multiscale imaging of organ-specific inflammation – our scientific concept
Dysregulated innate immune cell responses in vivo

Figure 1. Immune responses are initiated by various triggers and tightly regulated to ensure neutralisation or termination of the trigger and healing of tissues (black curve). However, immune responses can be suppressed as in the microenvironment of tumours (left switch). Dysregulation can result in overshooting or chronic inflammation. Further, even secondary hypo-responsive states can result such as in endotoxin tolerance (middle switch) with an inadequate immune response to a second inflammatory challenge (right switch, lower line). CRC inSight aims at better understanding how ‘thresholds’ that determine the outcome of inflammation are set. These act locally but require systemic innate immune cell dynamics and interaction to switch from initiation to resolution and from overshooting to chronic or even suppressed inflammation. Through understanding how immune tolerance and suppression states can be converted into appropriate and effective inflammation, e.g., through metabolic reprogramming (endotoxin tolerance) or cellular therapies (CAR gene-modified T cells), CRC inSight develops a robust translational perspective (re-activation, right switch, upper line).
Heterogeneity of organ-specific immune cell responses

Figure 2: Sterile (e.g. myocardial ischemia, top left), infectious (pneumonia, top right), autoimmune triggers (arthritis, bottom left) or invading tumours (bottom right) initiate or suppress an innate immune response, which involves different bone marrow-derived and tissue-resident immune cells that interact with each other, with the local endothelium (central circle) and with tissues. These cellular and molecular mechanisms define organ specificity of innate immune responses and its effect on organ function. CRC inSight develops and applies a novel multiscale imaging strategy to decipher these highly dynamic processes in vivo. CRC inSight covers the continuum ranging from the behaviour of individual immune, endothelial and tissueforming cells to functional consequences of inflammation in various organs, to entire organisms (mouse and man), and finally to use imaging to guide and tailor novel therapeutic approaches such as CAR T cells.
Multiscale imaging
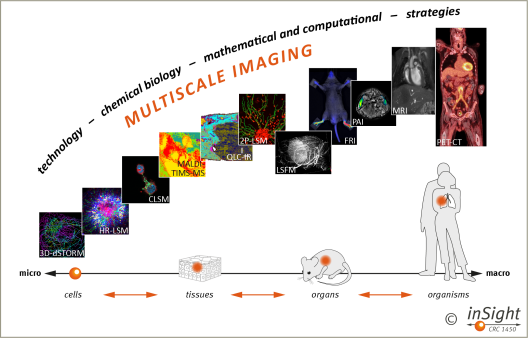
Figure 3: We integrate information from optical, intravital and whole-body imaging modalities through technological, chemical biology, mathematical and computational strategies to visualise the behaviour of individual cells, cells in tissues and organs and cells in whole organisms in disease models, and ultimately patients. Note that our multiscale imaging strategy allows for translation of information from basic research in disease models to clinical research in patients and vice versa. The different imaging modalities are all available in the CRC laboratories.
Technological strategies
These approaches focus on pushing the scale limits of individual imaging modalities. By advancing technology, we aim to achieve higher resolution and sensitivity, enabling more detailed visualization of immune processes across different scales.
Chemical biology strategies
Here, we are developing targeting and labelling methods that can be uniformly applied across multiple scales.
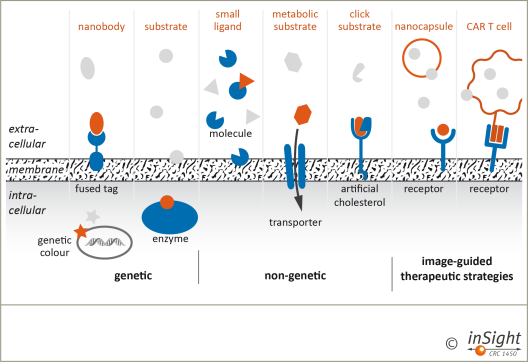
Figure 4: Genetic, non-genetic and image-guided targeting and labelling approaches for immune cells, bacteria, and the analysis of endothelial barriers in inflammatory scenarios will be developed and applied within CRC inSight. Labelled targeting units (orange) interact with extracellular, membrane-bound, or intracellular targets (green) providing specific imaging signals or molecular interventions.
Mathematical & computational strategies
These strategies involve the extraction and integration of information from imaging data obtained at different scales. By developing sophisticated mathematical and computational algorithms, we aim to identify patterns and features in multiscale imaging data, facilitating a deeper understanding of immune dynamics.

Figure 5: The mathematical/computational techniques used for bridging scales are not restricted to those projects in which they are developed. On the contrary, using joint software platforms and additional support by our university in our INF project to facilitate the application of the developed computational tools, all CRC projects are supposed to benefit from the novel machine learning, pattern recognition, interactive visualization and multiscale data analysis and management tools, which will help to create and test new biomedical hypotheses. Vice versa, these tools will be designed and adapted to optimally answer biomedical questions and to fully exploit the associated imaging and experimental data. We experience a mutual stimulation of the biomedical research (which is raised to a new level by the availability of multiscale data analysis) and the development of novel mathematical/computational concepts (which are triggered by biomedical ideas).
Deliverables and perspectives
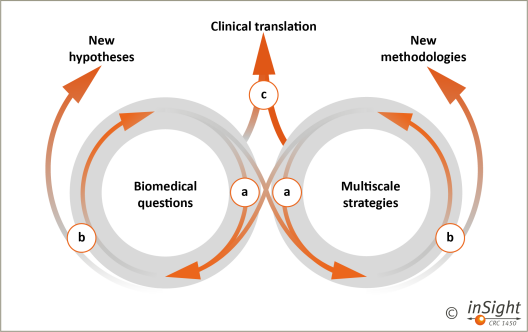
Figure 6: We study organ-specific immune responses in vivo using a novel and bridging multiscale imaging strategy. The concept will be established in phases: (a) a tight interaction between CRC projects driving biomedical questions (left) and those developing multiscale strategies (right) will set the basis for a dynamic interaction between models and methods, disciplines and individual research groups; (b) deliverables from the initial phase will be new hypotheses and methodologies, that will pose new questions and challenges for the respective discipline/field, but also drive other disciplines and fields (re-cycling and cross-over); long-term (c), all fields together aim at early clinical translation of novel biological concepts and imaging strategies to monitor and guide existing and emerging immune-modulatory therapies.

