Data Science
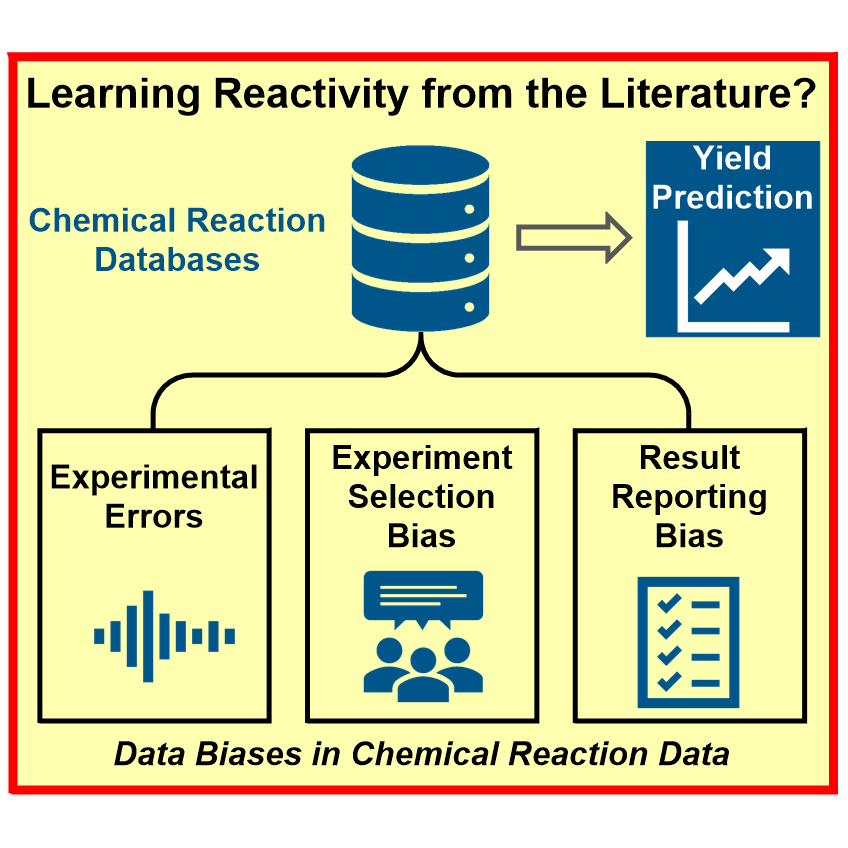
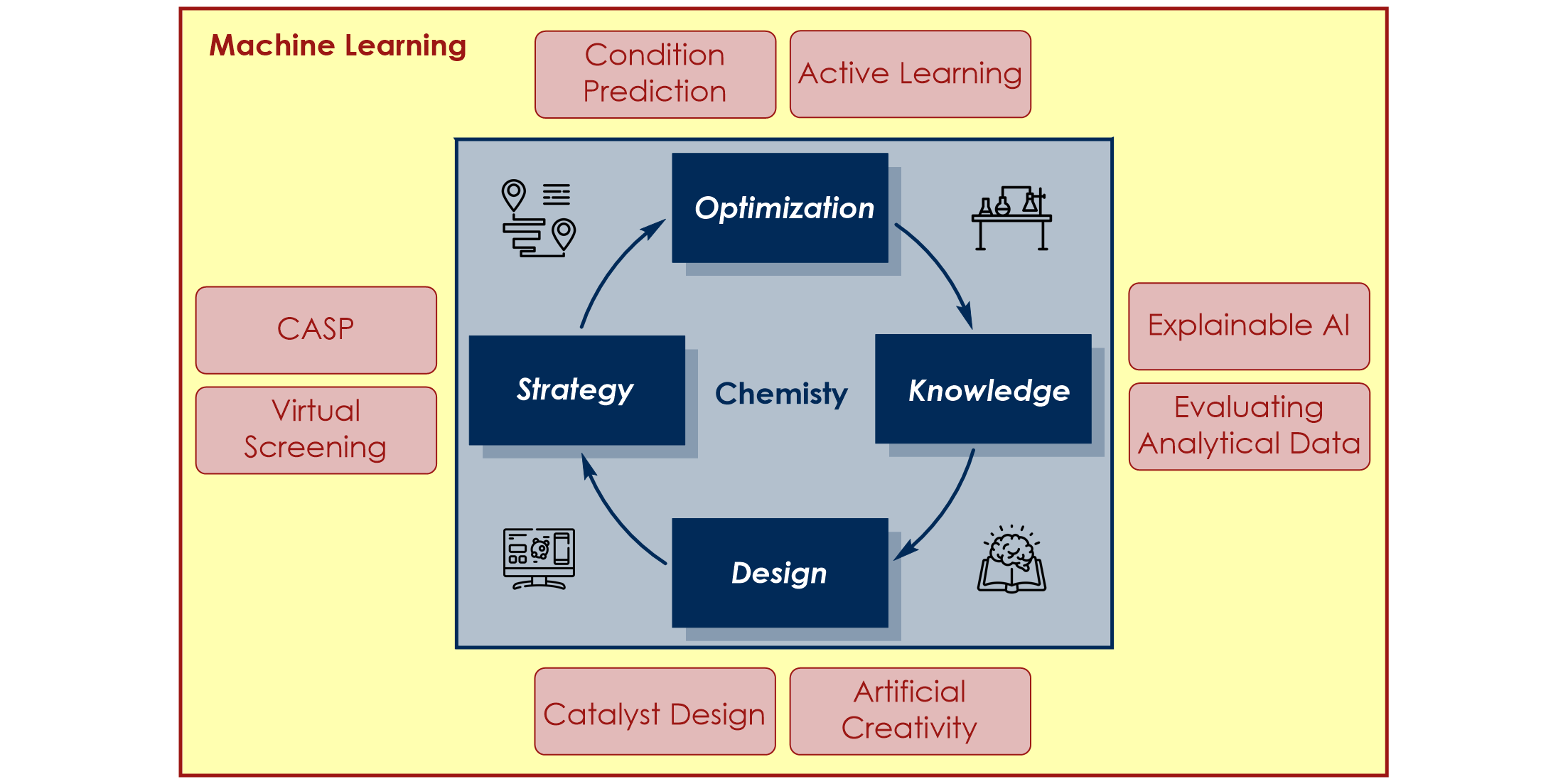
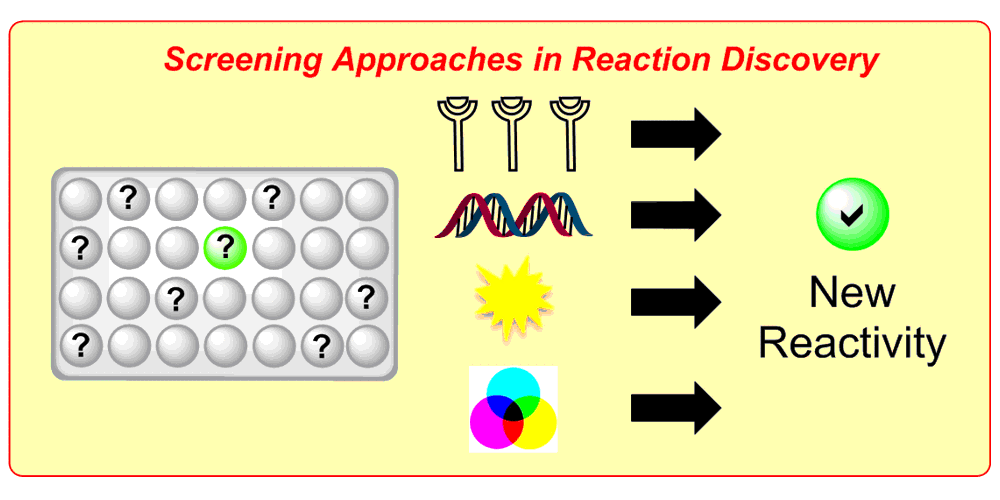
A major obstacle regarding the digitalization of organic synthesis is the availability of reaction-based data. To meet this challenge, we develop smart screening technologies for the efficient generation of high-quality data. By leveraging advanced machine learning approaches we extract valuable insights from this data and apply them to solve chemical challenges:
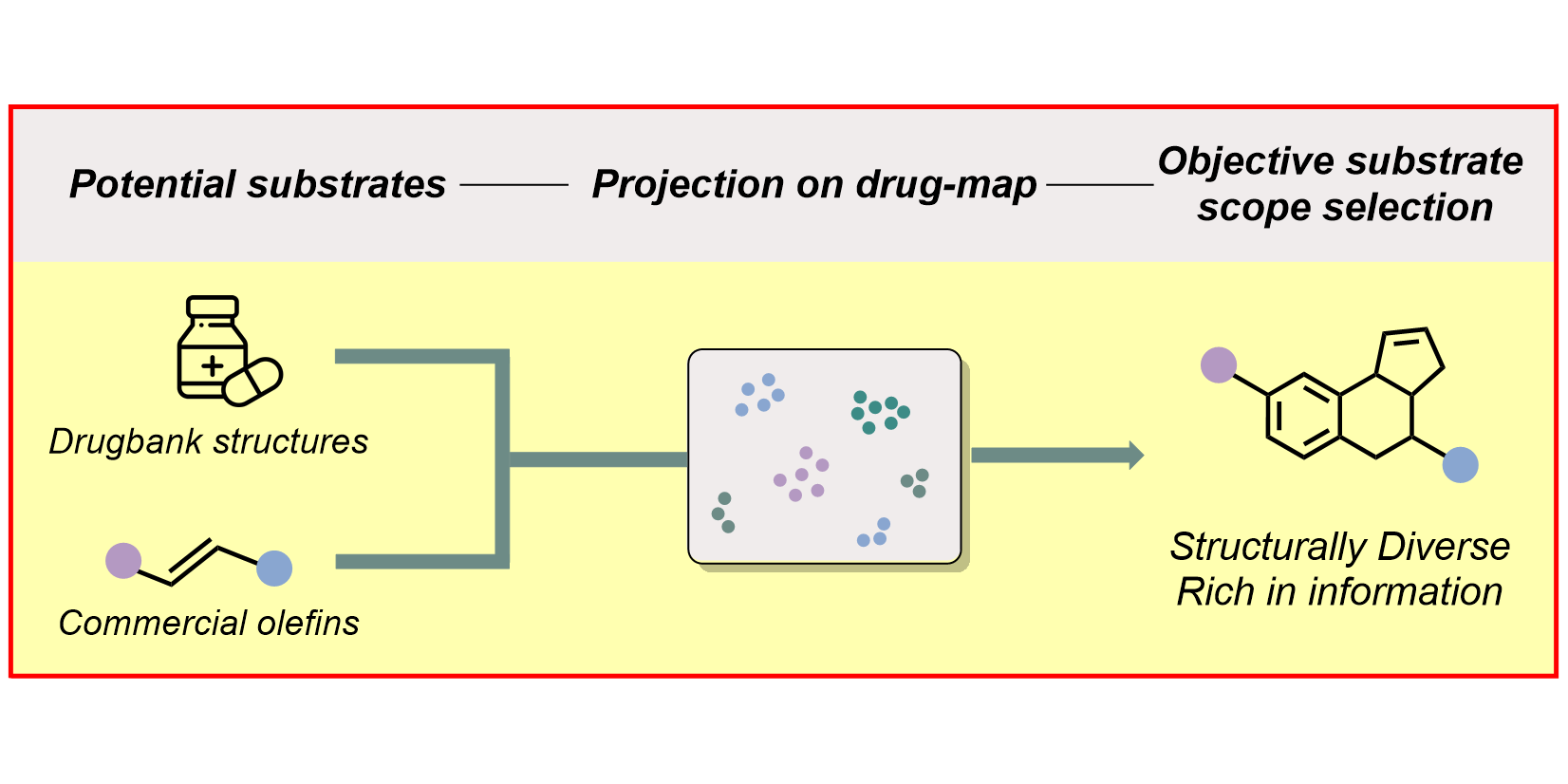
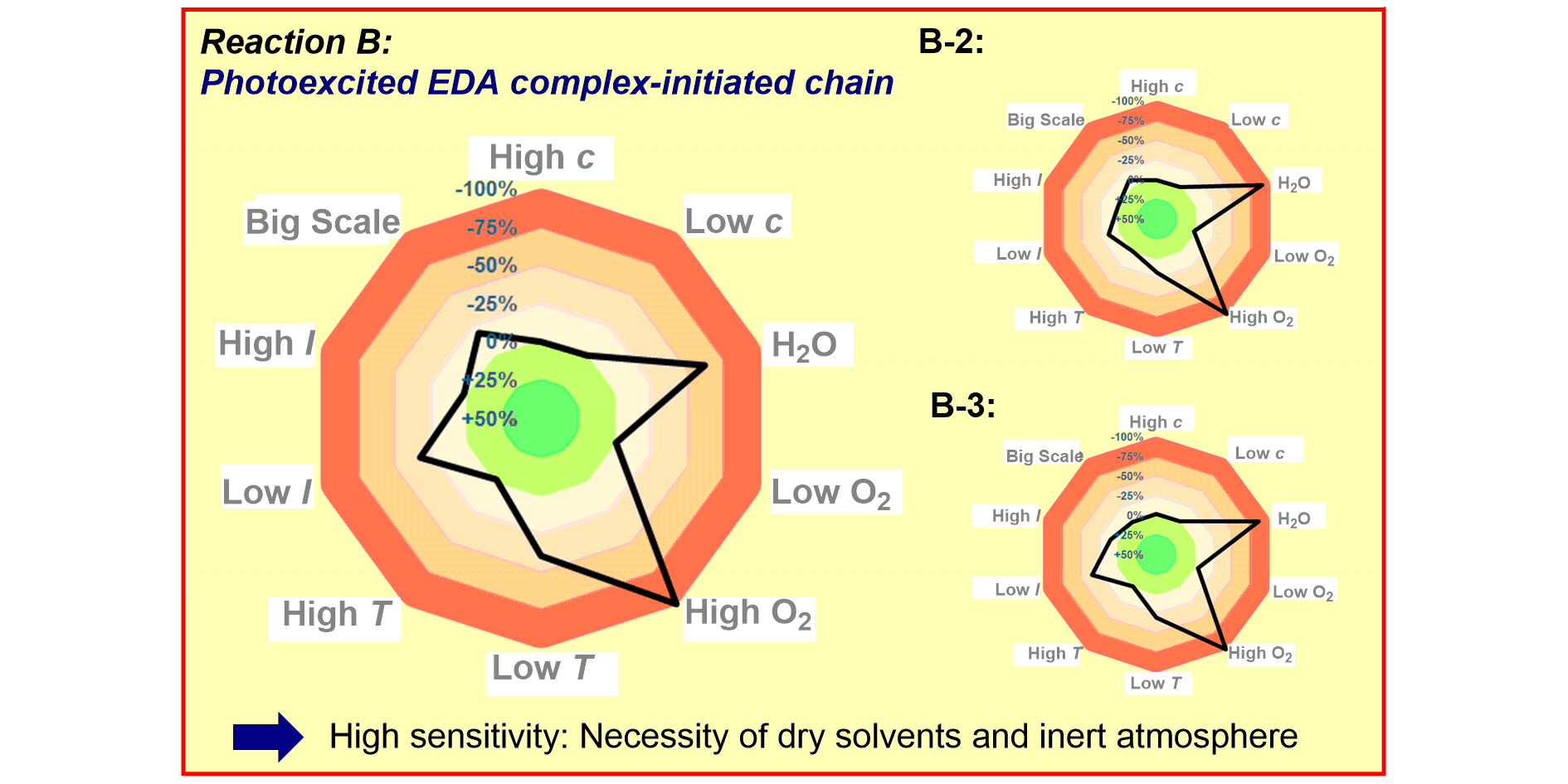
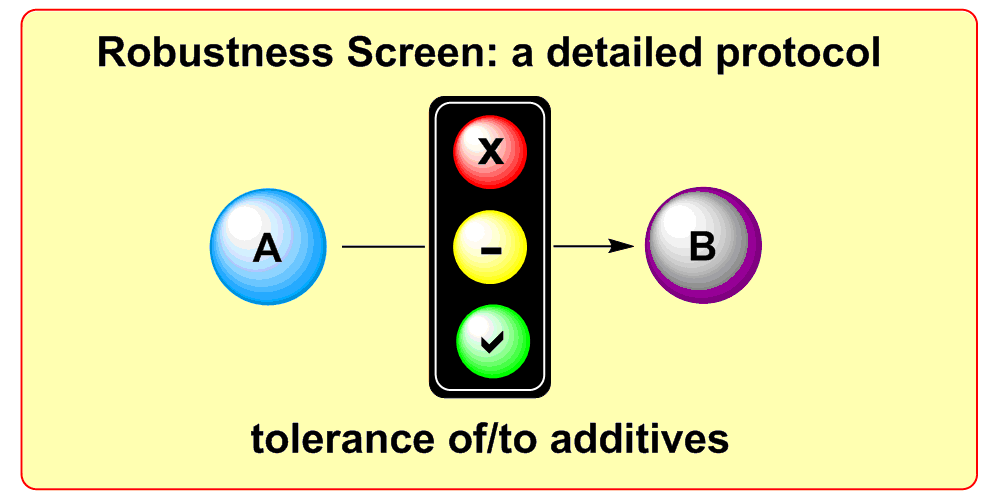
a) Reaction Assessment: For the rapid assessment of chemical reactions, we developed an additive-based robustness screen[1] to evaluate functional group tolerance and a condition-based sensitivity screen[2] to reduce reproducibility issues. Furthermore, to increase the informativeness in a reaction’s scope, we developed a data-driven substrate selection strategy.[3]
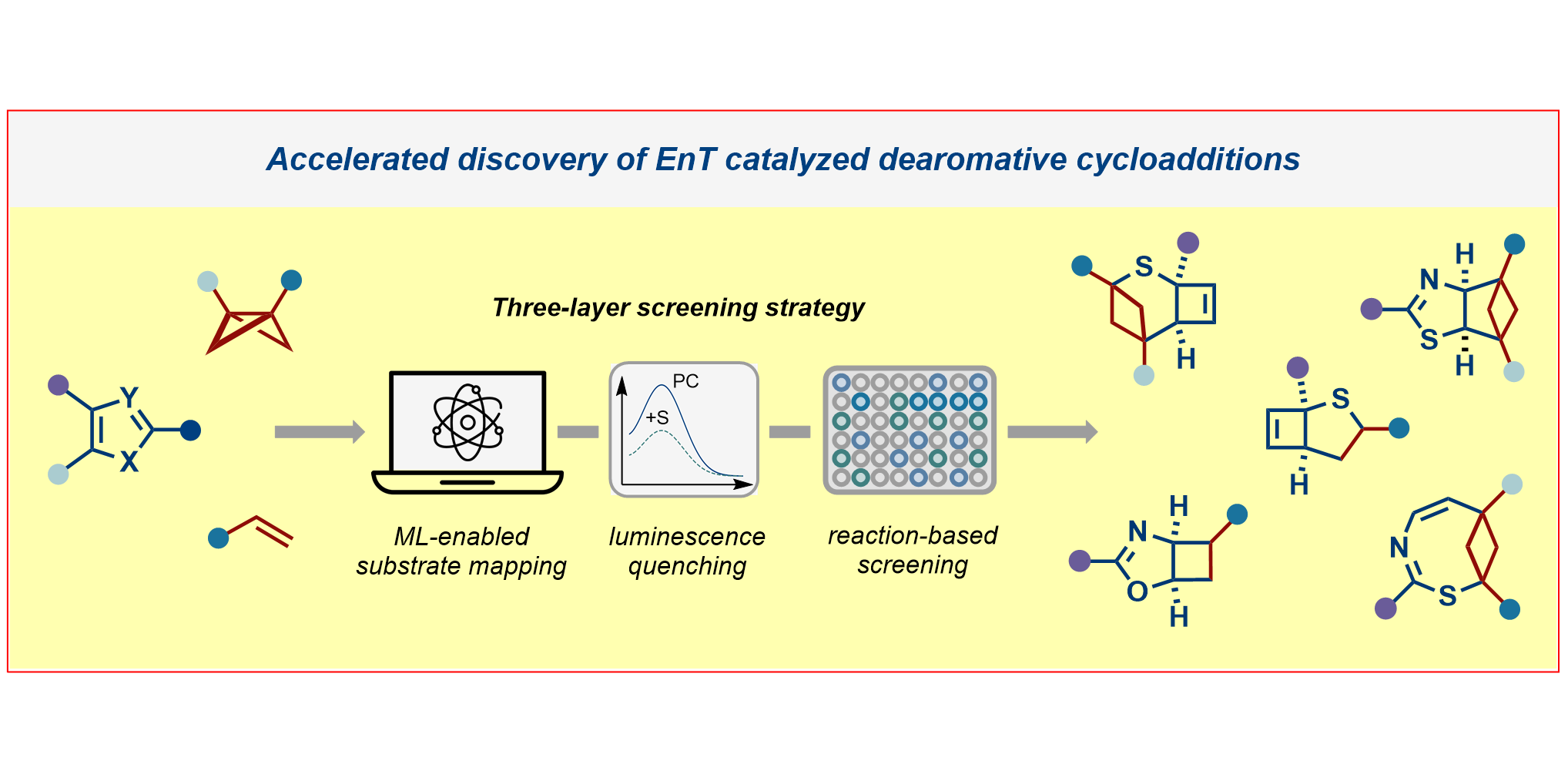
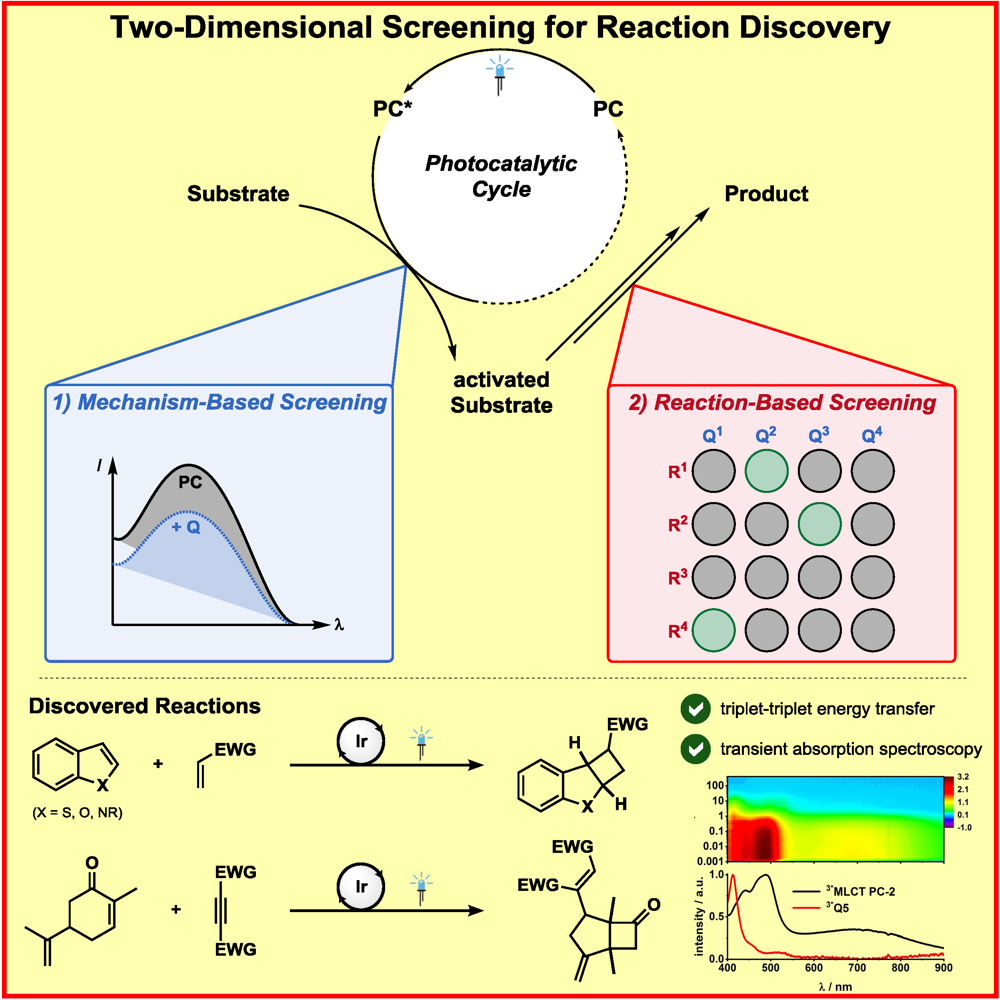
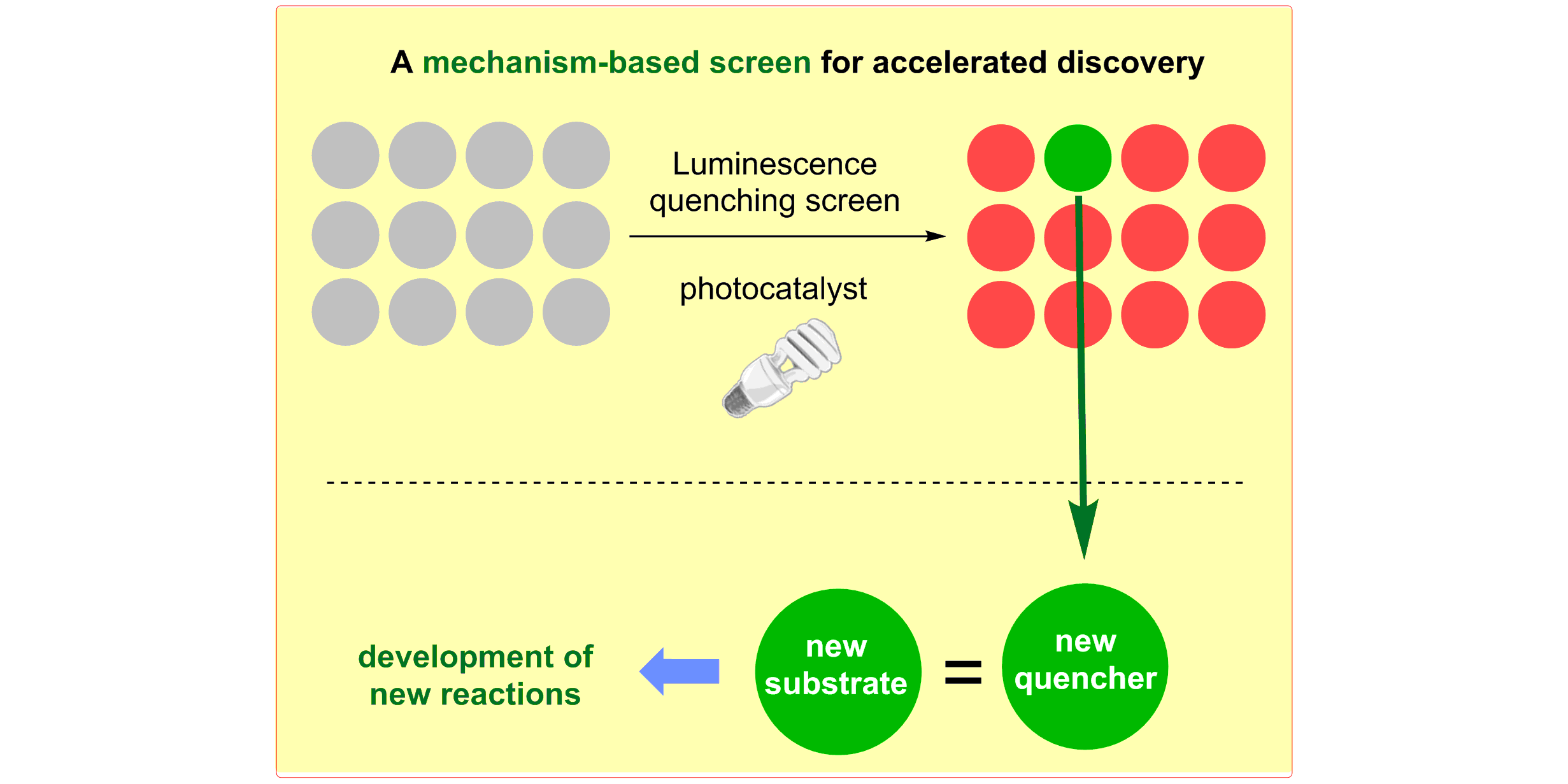
b) Accelerated Discovery: A mechanism-based screening strategy based on luminescence quenching[4] allowed for the discovery of various photochemical transformations. The serendipitous findings could further be enhanced via a two-dimensional screening approach.[5]
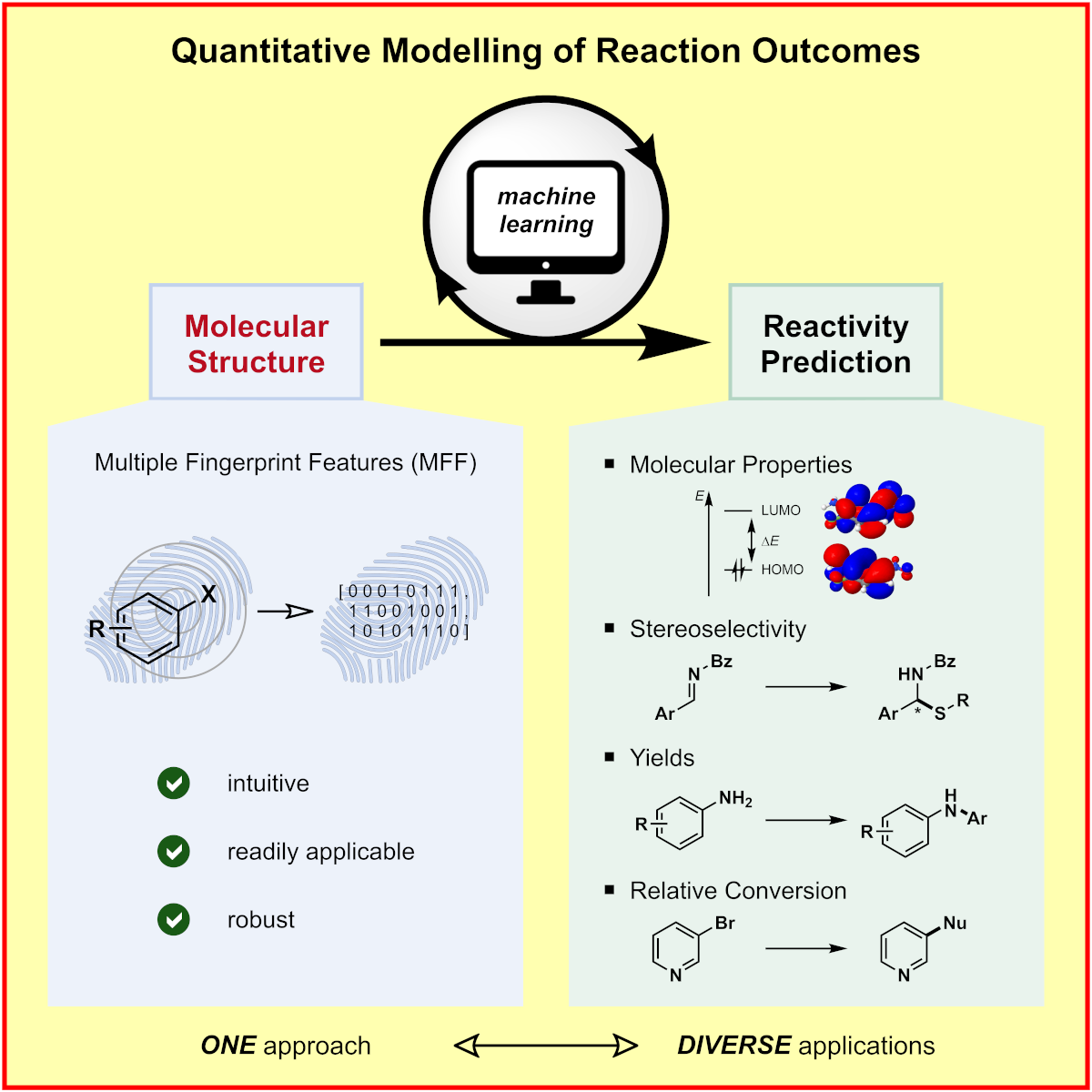
c) Molecular Machine Learning: We developed a genetic algorithm yielding problem-specific and interpretable molecular representations[6] which can be used for quantitative prediction of reactivity.[7] Moreover, we utilize high-throughput virtual screening for accelerated reaction discovery.[8]
[1] K. D. Collins, F. Glorius, Nature Chem. 2013, 5, 597. [2] L. Pitzer, F. Schäfers, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 8572. [3] D. Rana, P. M. Pflüger, N. P. Hölter, G. Tan, F. Glorius, ACS Cent. Sci. 2024, 10, 899. [4] M. N. Hopkinson, A. Gómez‐Suárez, M. Teders, B. Sahoo, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 4361. [5] F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi, F. Glorius, Chem 2019, 5, 339. [6] P. M. Pflüger, M. Kühnemund, F. Katzenburg, H. Kuchen, F. Glorius, Chem 2024, 10, 1391. [7] F. Sandfort, F. Strieth-Kalthoff, M. Kühnemund, C. Beecks, F. Glorius, Chem 2020, 6, 1379. [8] L. Schlosser, D. Rana, P. M. Pflüger, F. Katzenburg, F. Glorius, J. Am. Chem. Soc. 2024, 146, 13266.
Please click on the graphical abstracts to come to the original publication
D. Rana, C. Hümpel, R. Laskar, L. Schlosser, S. Korgitzsch, S. Dutta, C. G. Daniliuc, F. Glorius,
Accelerated Discovery of Energy Transfer-Catalyzed Dearomative Cycloadditions through a Data-Driven Three-Layer Screening Strategy,
J. Am. Chem. Soc. 2025, 147, 28359-2836.
J. L. Tyler,* D. Trauner,* F. Glorius,*
Reaction Development: A Student's Checklist,
Chem. Soc. Rev. 2025, 54, 3272-3292.
F. Katzenburg, F. Boser, F. R. Schäfer, P. M. Pflüger, F. Glorius,
Calibration-free quantification and automated data analysis for high-throughput reaction screening,
Digit. Discov. 2025, 4, 384-392.
F. Schäfer, L. Lückemeier, F. Glorius,
Improving reproducibility through condition-based, sensitivity assessments: application, advancement, and prospect,
Chem. Sci. 2024, 15, 14548-14555.
S. P. Schmid,§ L. Schlosser,§ F. Glorius,* K. Jorner,*
Catalysing (organo-) catalysis: Trends in the application of machine learning to enantioselective organocatalysis,
Beilstein J. Org. Chem. 2024, 20, 2280-2304.
§ These authors contributed equally.
L. Schlosser, D. Rana, P. Pflüger, F. Katzenburg, F. Glorius,*
EnTdecker – A machine learning-based platform for guiding substrate discovery in energy transfer catalysis,
J. Am. Chem. Soc. 2024, 146, 13266-13275.
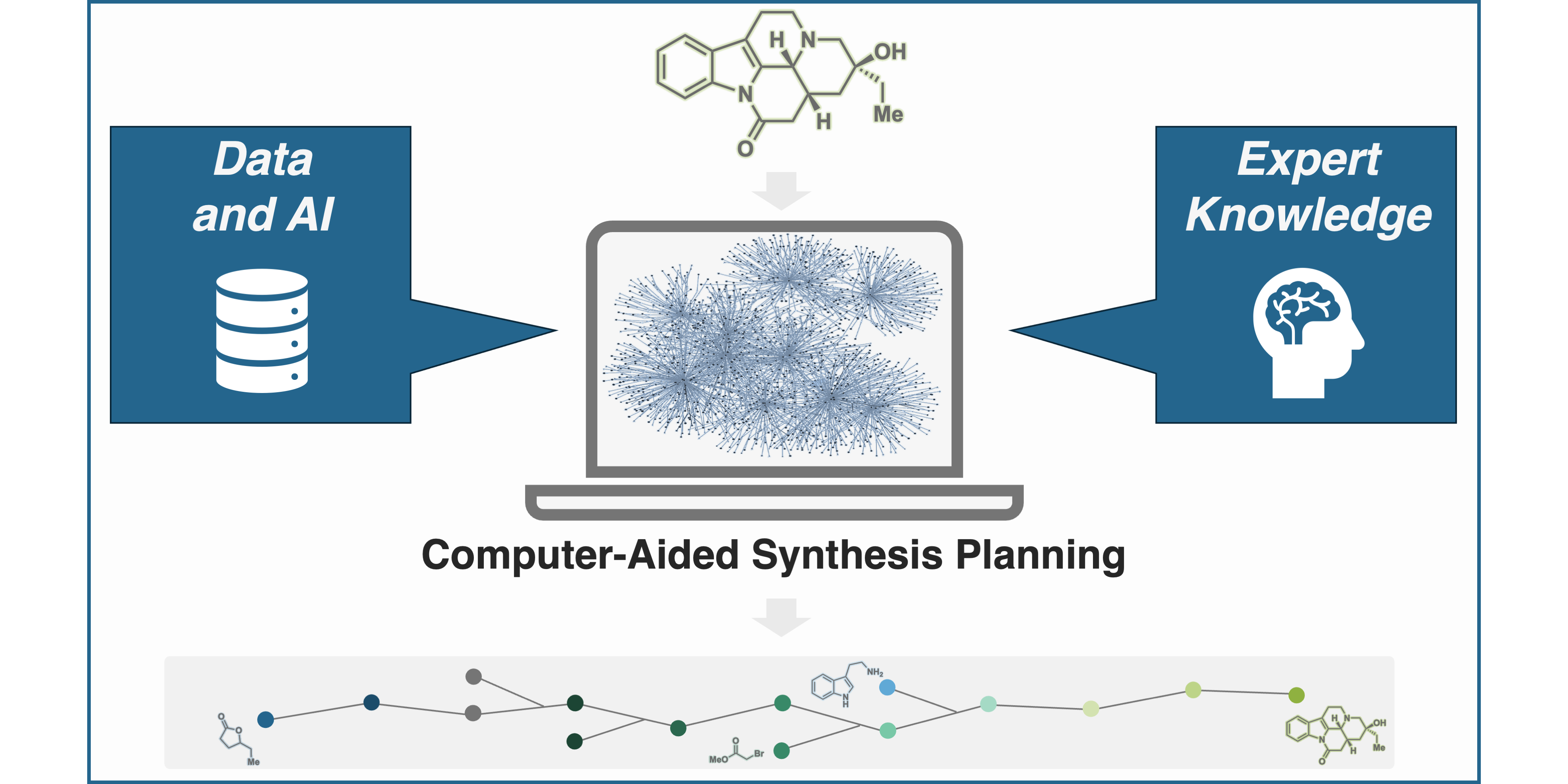
F. Strieth-Kalthoff,§ S. Szymkuc,§ K. Molga,§ A. Aspuru-Guzik, F. Glorius,* B. A. Grzybowski,*
Artificial Intelligence for Retrosynthetic Planning Needs Both Data and Expert Knowledge,
J. Am. Chem. Soc. 2024, 146, 11005-11017.
§ These authors contributed equally.
D. Rana, P. M. Pflüger, N. P. Hölter, G. Tan, F. Glorius,
Standardizing Substrate Selection: A Strategy toward Unbiased Evaluation of Reaction Generality,
ACS Cent. Sci. 2024, 10, 899-906.
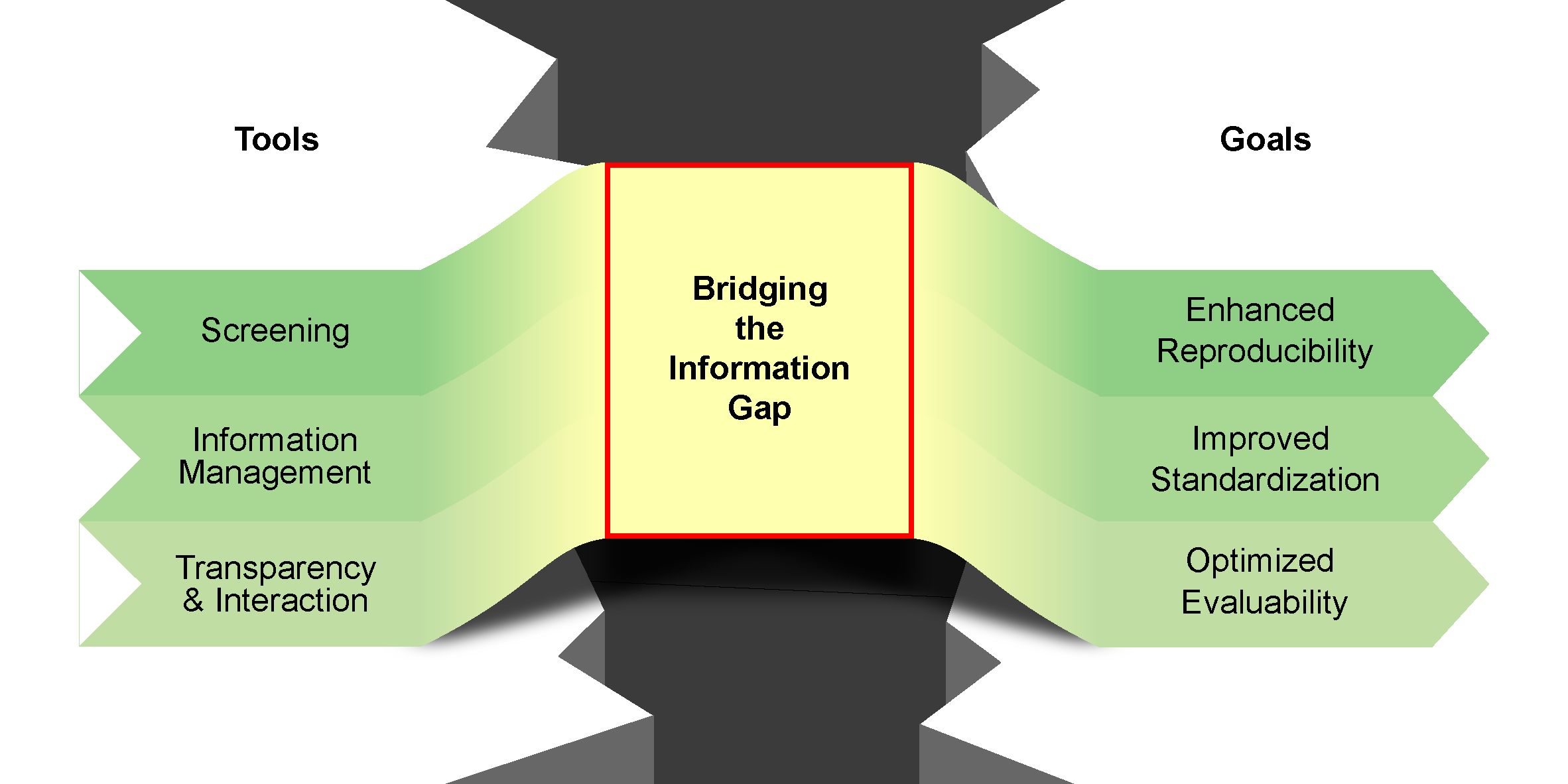
M. L. Schrader,§ F. R. Schäfer,§ F. Schäfers,§ F. Glorius,
Bridging the information gap in organic chemical reactions,
Nat. Chem. 2024, 16, 491-498.
§ These authors contributed equally.
P. M. Pflüger,§ M. Kühnemund,§ F. Katzenburg,§ H. Kuchen, F. Glorius,
An evolutionary algorithm for interpretable molecular representations,
Chem 2024, 10, 1391-1405.
§ These authors contributed equally; selected for cover image.
F. Strieth-Kalthoff,§ F. Sandfort,§ M. Kühnemund, F. R. Schäfer, H. Kuchen, F. Glorius,
Machine Learning for Chemical Reactivity: The Importance of Failed Experiments,
Angew. Chem. Int. Ed. 2022, 61 (29), e202204647; Angew. Chem. 2022, 134 (29), e202204647.
P. M. Pflüger, F. Glorius,
Molecular Machine Learning: The Future of Synthetic Chemistry?,
Angew. Chem. Int. Ed. 2020, 59, 18860-18865; Angew. Chem. 2020, 132, 19020-19025.
F. Strieth-Kalthoff, F. Sandfort, M. H. S. Segler, F. Glorius,
Machine Learning the Ropes: Principles, Applications and Directions in Synthetic Chemistry,
Chem. Soc. Rev. 2020, 49, 6154-6168.
F. Sandfort,§ F. Strieth-Kalthoff,§ M. Kühnemund,§ C. Beecks, F. Glorius,*
A Structure-Based Platform for Predicting Chemical Reactivity,
Chem 2020, 6, 1379-1390.
ChemRxiv (Preprint) 2019. DOI
§ All three authors contributed equally.
L. Anhäuser, M. Teders, A. Rentmeister,* F. Glorius,*
Bio-additive-based screening: toward evaluation of the biocompatibility of chemical reactions,
Nature Protoc. 2019, 14, 2599-2626.
F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi,* F. Glorius,*
Discovery of Unforeseen Energy-Transfer-Based Transformations Using a Combined Screening Approach,
Chem 2019, 5, 2183-2194.
L. Pitzer,§ F. Schäfers,§ F. Glorius,
Rapid Assessment of the Reaction Condition-Based Sensitivity of Chemical Transformations,
Angew. Chem. Int. Ed. 2019, 58, 8572-8576; Angew. Chem. 2019, 131, 8660-8664.
§ Both authors contributed equally.
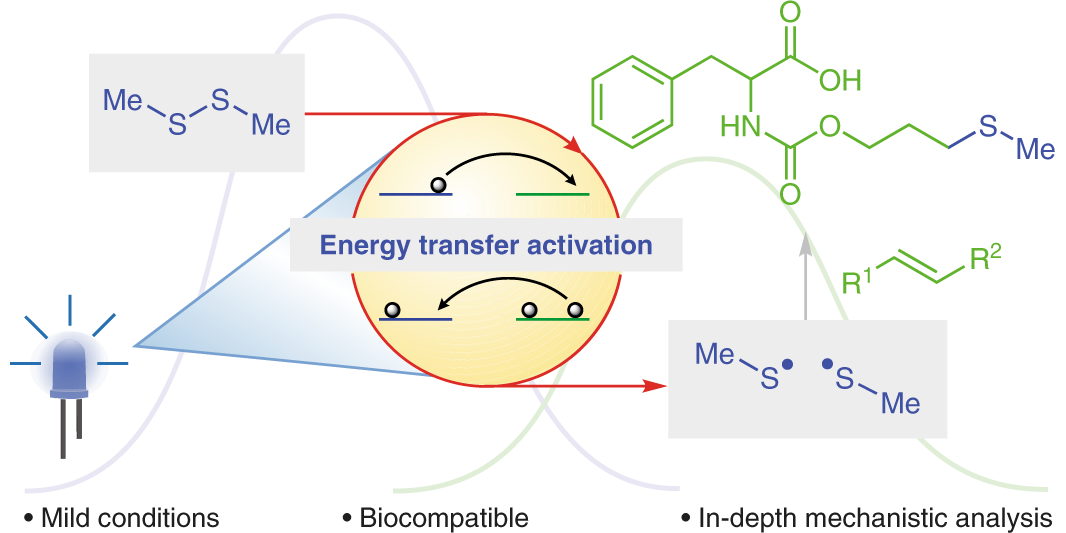
M. Teders, C. Henkel, L. Anhäuser, F. Strieth-Kalthoff, A. Goméz-Suárez, R. Kleinmans, A. Kahnt, A. Rentmeister, D. M. Guldi,* F. Glorius,*
The energy-transfer-enabled biocompatible disulfide–ene reaction,
Nat. Chem. 2018, 10, 981-988.
T. Gensch§, M. Teders§, F. Glorius,
An Approach to Comparing the Functional Group Tolerance of Reactions,
J. Org. Chem. 2017, 82, 9154-9159.
§ Both authors contributed equally.
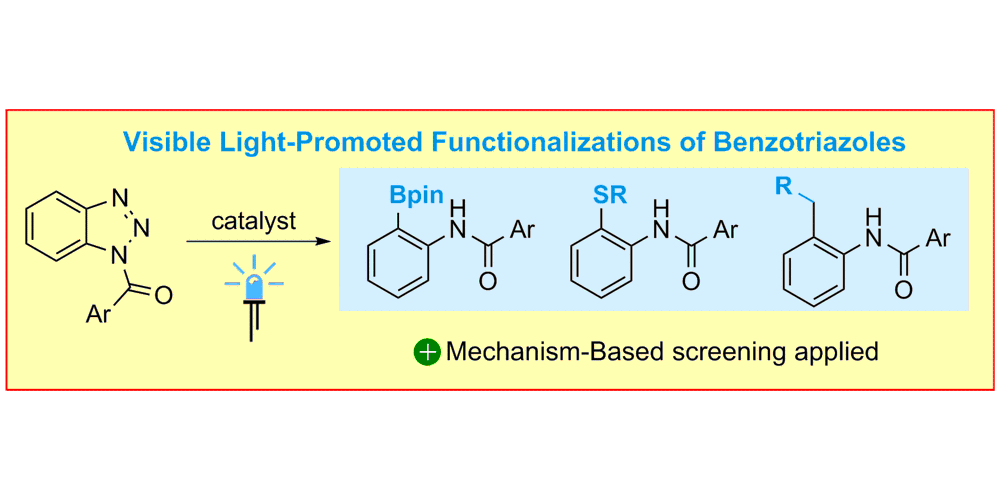
M. Teders, A. Gómez-Suárez§, L. Pitzer§, M. N. Hopkinson, F. Glorius,
Diverse Visible-Light-Promoted Functionalizations of Benzotriazoles Inspired by Mechanism-Based Luminescence Screening,
Angew. Chem. Int. Ed. 2017, 56, 902-906; Angew. Chem. 2017, 129, 921-925.
M. N. Hopkinson, A. Gomez-Suarez, M. Teders, B. Sahoo, F. Glorius,
Accelerated Discovery in Photocatalysis using a Mechanism-Based Screening Method,
Angew. Chem. Int. Ed. 2016, 55, 4361-4366; Angew. Chem. 2016, 128, 4434-4439.
K. D. Collins, T. Gensch, F. Glorius,
Contemporary screening approaches to reaction discovery and development,
Nat. Chem. 2014, 6, 859–871.
K. D. Collins, A. Rühling, F. Glorius,
Application of a Robustness Screen for the evaluation of synthetic organic methodology,
Nat. Protoc. 2014, 9, 1348-1353.
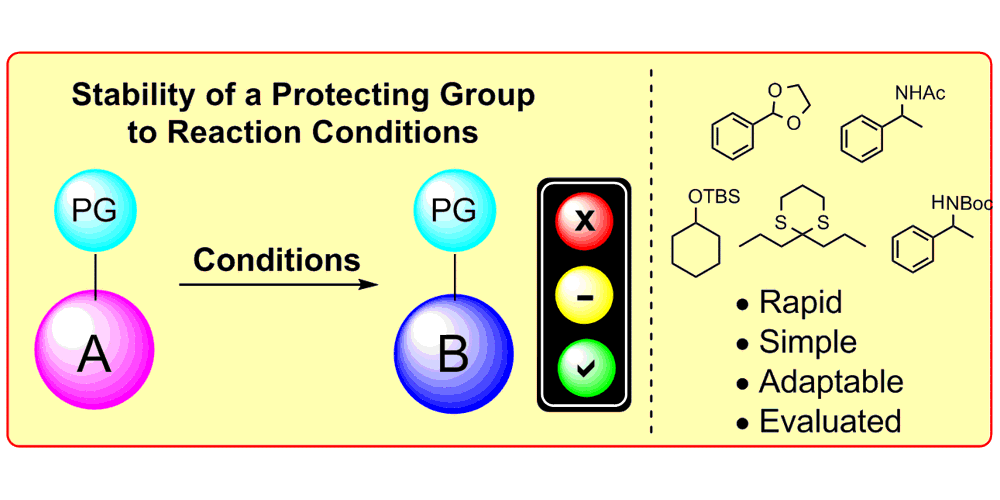
K. D. Collins,* A. Rühling, F. Lied, F. Glorius,*
Rapid Assessment of Protecting Group Stability Using a Robustness Screen,
Chem. Eur. J. 2014, 20, 3800-3805.
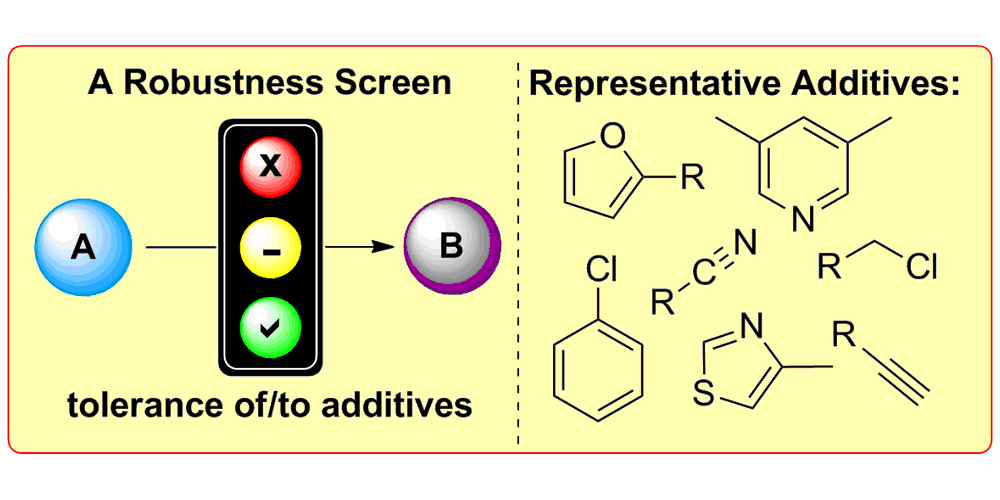
K. D. Collins, F. Glorius,
A Robustness Screen for the Rapid Assessment of Chemical Reactions,
Nature Chem. 2013, 5, 597-601.
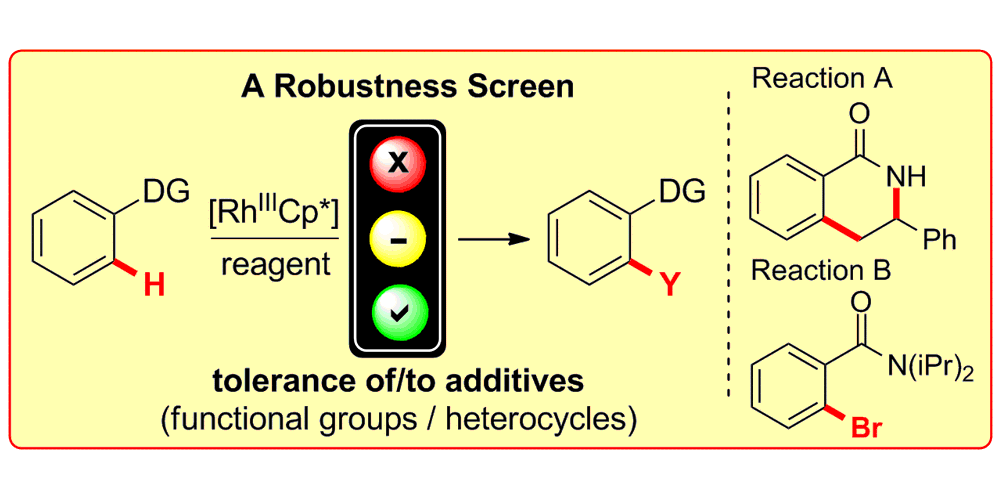
K. D. Collins, F. Glorius,
Employing a Robustness Screen: Rapid Assessment of Rhodium(III)-catalysed C-H Activation Reactions,
Tetrahedron 2013, 69, 7817-7825.