Arene Hydrogenation
Arene hydrogenation is a powerful tool to transform commercially available, planar and easy to modify starting materials to more complex, three dimensional building blocks. These can expand the scope of possible drug candidates in pharmaceutical research. Our group addressed a major limitation of functional group tolerance by utilizing a novel catalyst system (to the right).[1-4] Utilizing this bench stable Rh carbene complex as a precursor and obtaining Rh nanoparticles in situ as the active catalyst species, a variety of (hetero)arenes could be transformed to their corresponding saturated cycles under hydrogen pressure.[5] Future work in this area will include variations of the process to obtain trans-selectivity, allowing for lower hydrogen pressure to be used and enabling an asymmetric hydrogenation.
[1] M. P. Wiesenfeldt, Z. Nairoukh, W. Li, F. Glorius, Science 2017, 357, 908. [2] M. Wollenburg, D. Moock, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 6549. [3] M. P. Wiesenfeldt, T. Knecht, C. Schlepphorst, F. Glorius, Angew. Chem. Int. Ed. 2018, 57, 8297. [4] Z. Nairoukh, M. Wollenburg, C. Schlepphorst, K. Bergander, F. Glorius, Nature Chem. 2019, 11, 264. [5] V. Lavallo, Y. Canac, C. Präsang, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2005, 44, 5705; Y. Wei, B. Rao, X. Cong, X. Zeng, J. Am. Chem. Soc. 2015, 137, 9250.
Please click on the graphical abstracts to come to the original publication
M. Pierau,§ M. J. Karrasch,§ P. Hartmann, C. G. Daniliuc, A. Hamza,* F. Glorius,*
Direct access to chiral nitrogen-rich (semi-)saturated heterocycles,
Chem 2025, 12, 102817.
§ These authors contributed equally.<
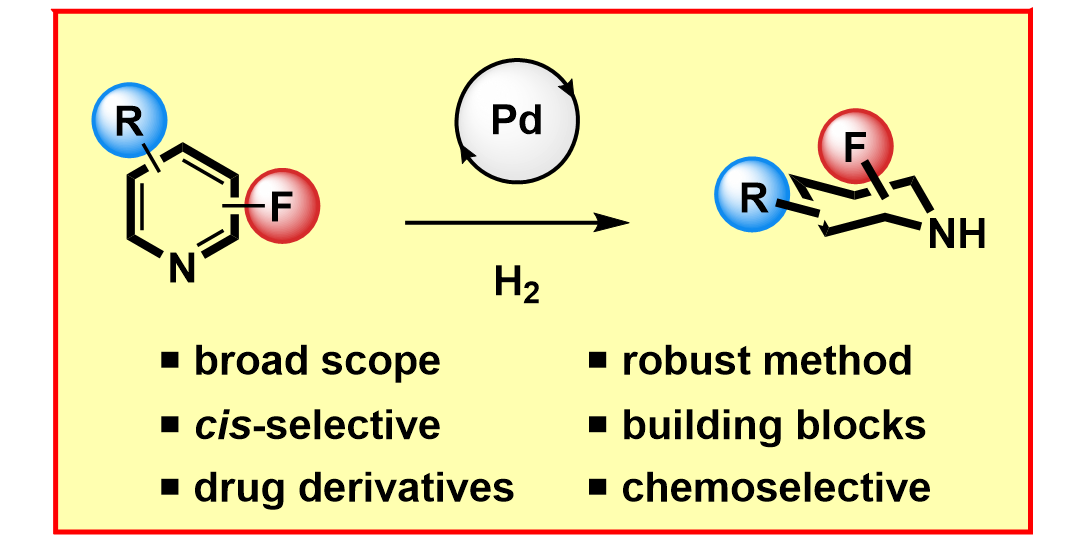
F. Zhang, H. Sekhar Sasmal,§ D. Rana,§ F. Glorius,
Switchable and Chemoselective Arene Hydrogenation for Efficient Late Stage Applications,
J. Am. Chem. Soc. 2024, 146, 18682-18688.
§ These authors contributed equally.
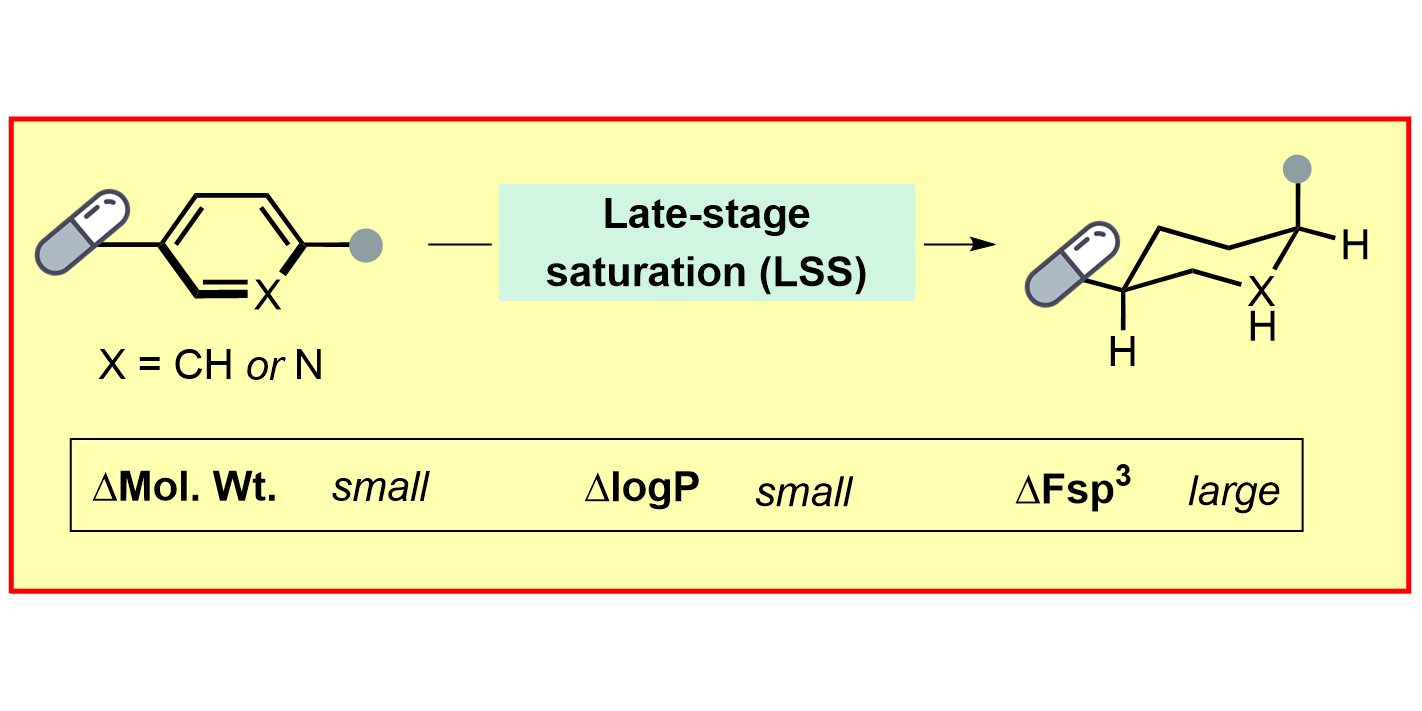
D.-H. Liu,§ P. M. Pflüger,§ A. Outlaw,§ L. Lückemeier, F. Zhang, C. Regan, H. Rashidi Nodeh, T. Cernak,* J. Ma,* F. Glorius,*
Late-Stage Saturation of Drug Molecules,
J. Am. Chem. Soc. 2024, 146, 11866-11875.
§ These authors contributed equally.
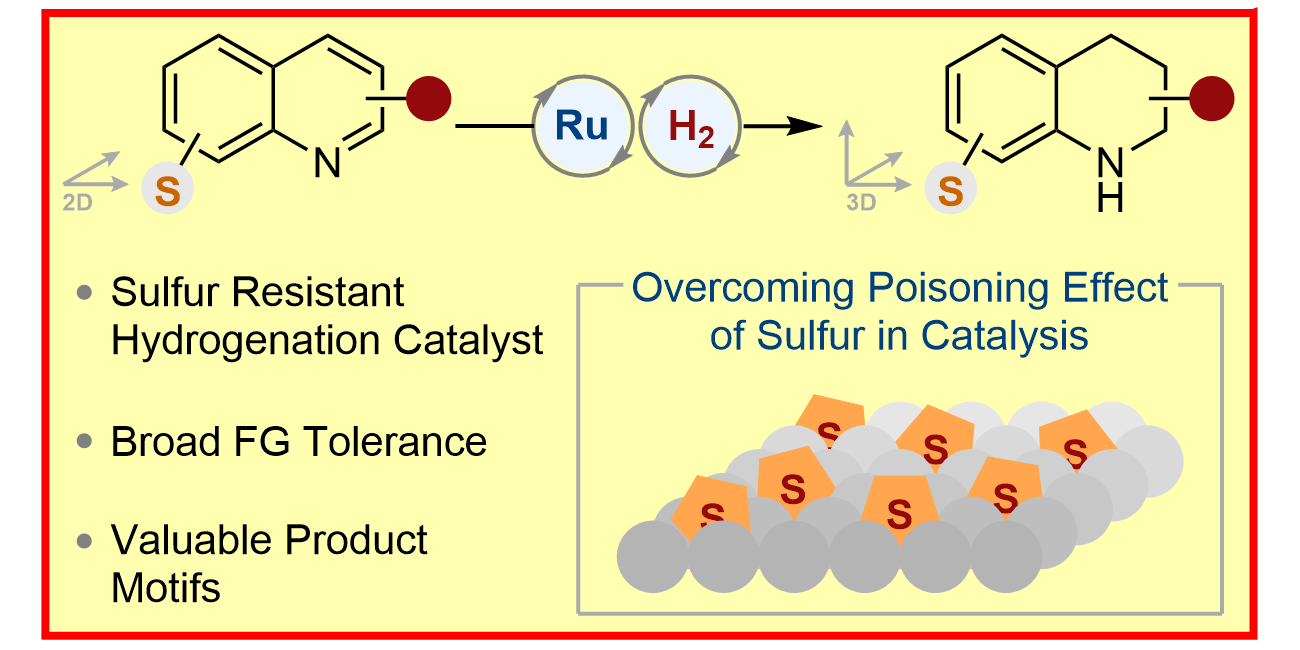
L. Lückemeier,§ T. De Vos,§ L. Schlichter, C. Gutheil, C. G. Daniliuc, F. Glorius,
Chemoselective Heterogeneous Hydrogenation of Sulphur Containing Quinolines Under Mild Conditions,
J. Am. Chem. Soc. 2024, 146, 5864-5871.
§ These authors contributed equally.
L. Lückemeier,§ M. Pierau,§ F. Glorius,
Asymmetric arene hydrogenation: towards sustainability and application,
Chem. Soc. Rev. 2023, 52, 4996-5012.
§ These authors contributed equally
F. Zhang, H. S. Sasmal, C. G. Daniliuc, F. Glorius,
Ru-NHC-Catalyzed Asymmetric, Complete Hydrogenation of Indoles and Benzofurans: One Catalyst with Dual Function,
J. Am. Chem. Soc. 2023, 145, 15695-15701.
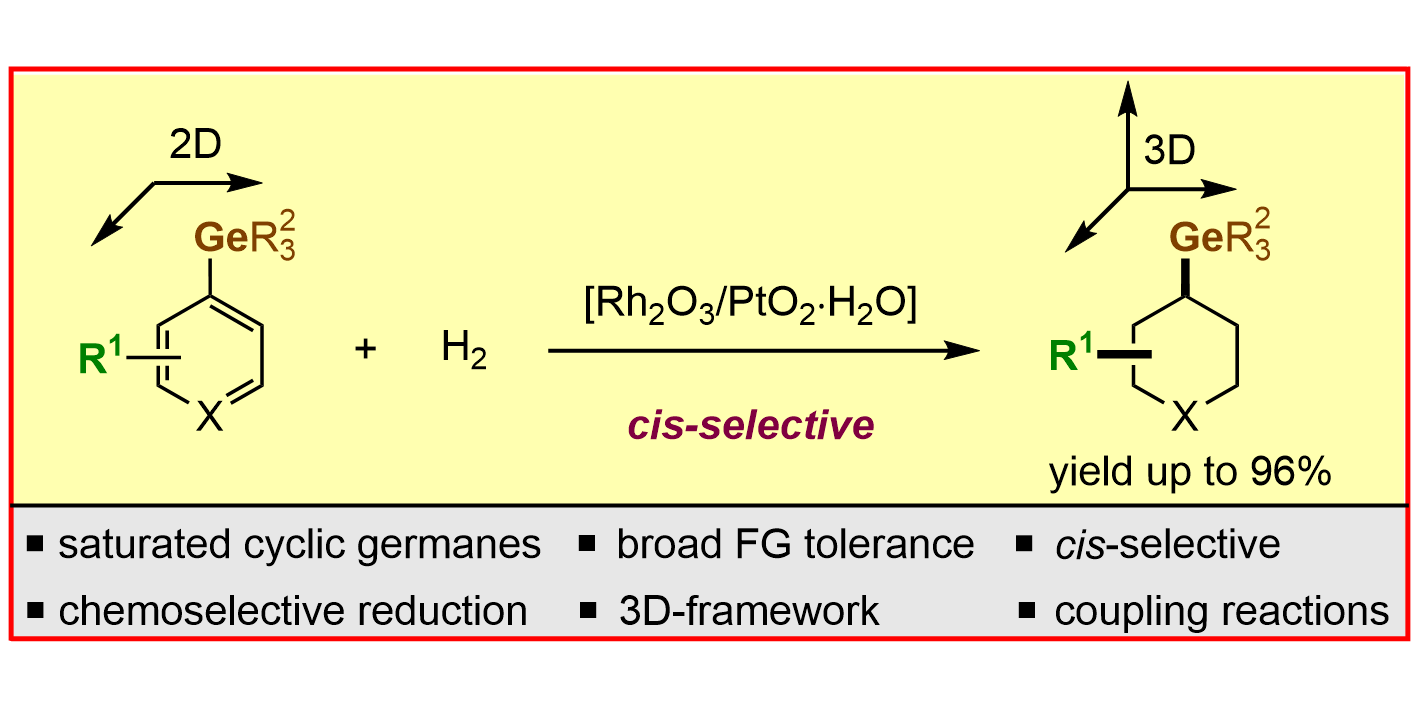
A. Kaithal, H. Sekhar Sasmal, S. Dutta, F. Schäfer, L. Schlichter, F. Glorius,
cis-Selective Hydrogenation of Aryl Germanes: A Direct Approach to Access Saturated Carbo- and Heterocyclic Germanes,
J. Am. Chem. Soc. 2023, 145, 4109-4118.
A. Kaithal, T. Wagener, P. Bellotti, C. G. Daniliuc, L. Schlichter, F. Glorius,
Access to Unexplored 3D Chemical Space: cis-Selective AreneHydrogenation for the Synthesis of Saturated Cyclic Boronic Acids,
Angew. Chem. Int. Ed. 2022, 61(32), e202206687; Angew. Chem. 2022, 134 (32), e202206687.
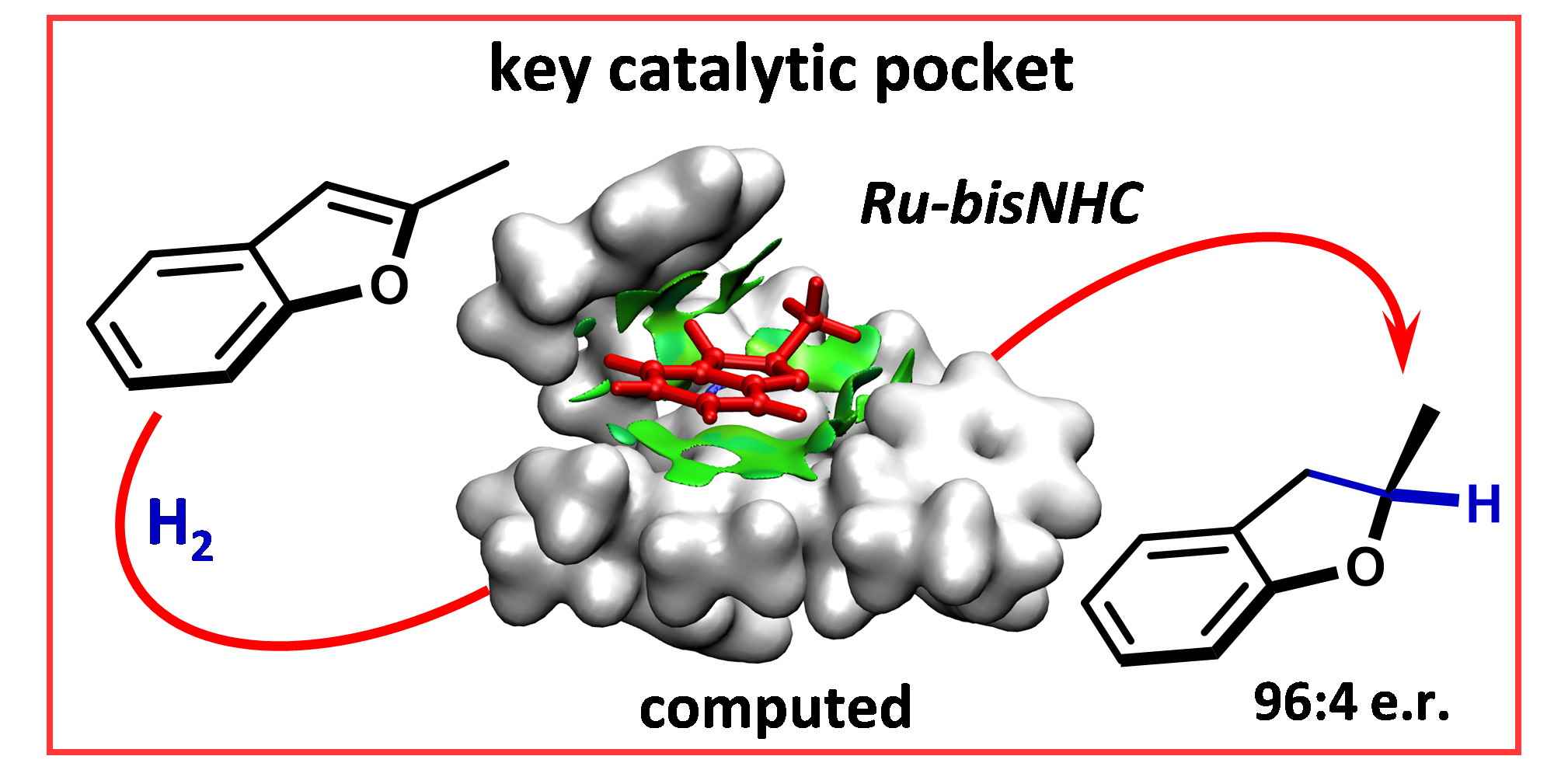
A. Hamza,* D. Moock, C. Schlepphorst, J. Schneidewind, W. Baumann, F. Glorius*,
Unveiling a key catalytic pocket for the ruthenium NHC-catalysed asymmetric heteroarene hydrogenation,
Chem. Sci. 2022, 13, 985-995.
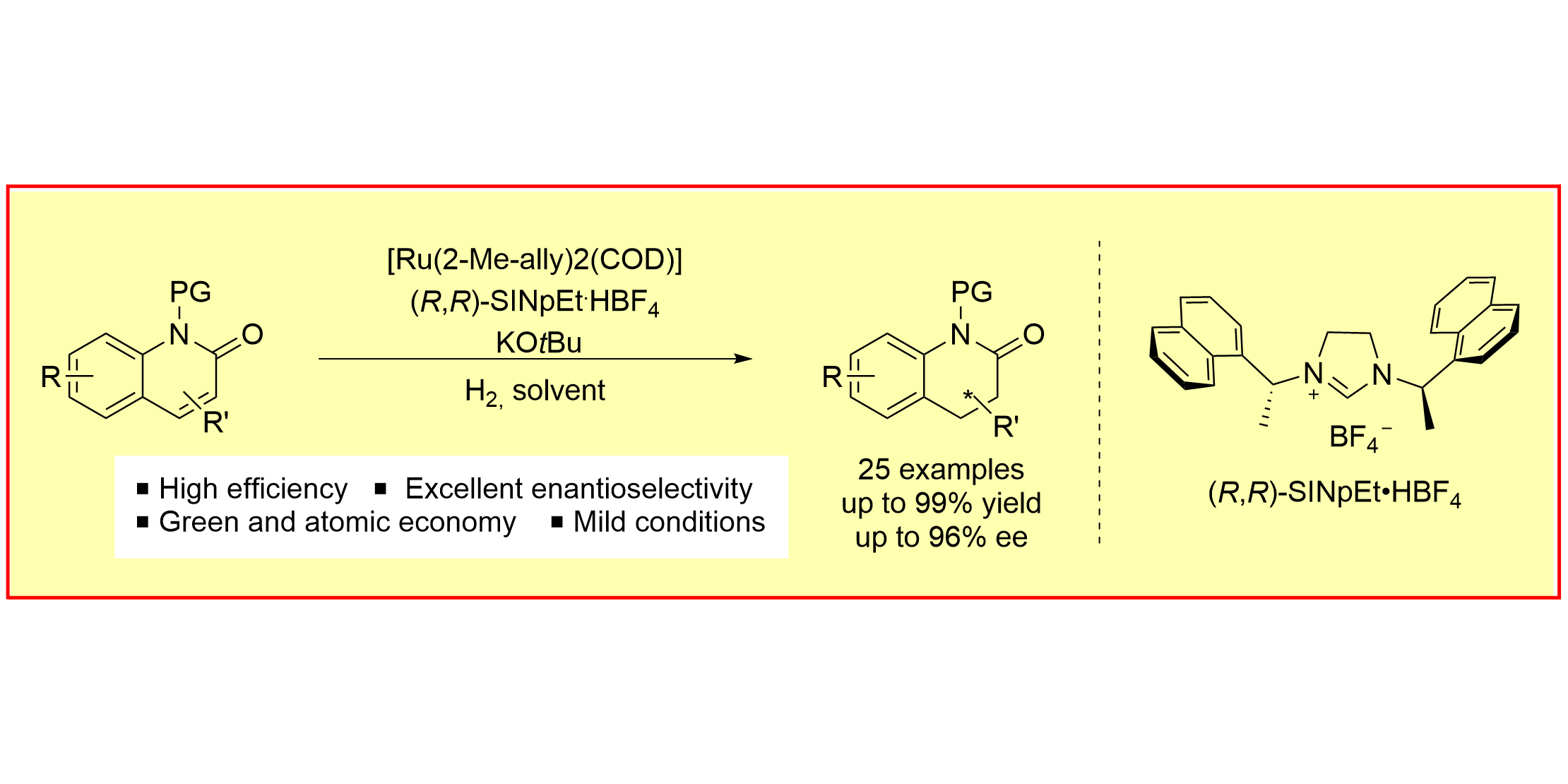
T. Hu, L. Lückemeier, C. Daniliuc, F. Glorius,
Ru-NHC-Catalyzed Asymmetric Hydrogenation of 2-Quinolones to Chiral 3,4-Dihydro-2-Quinolones,
Angew. Chem. Int. Ed. 2021, 60, 23193-23196; Angew. Chem. 2021, 133, 23377-23381.
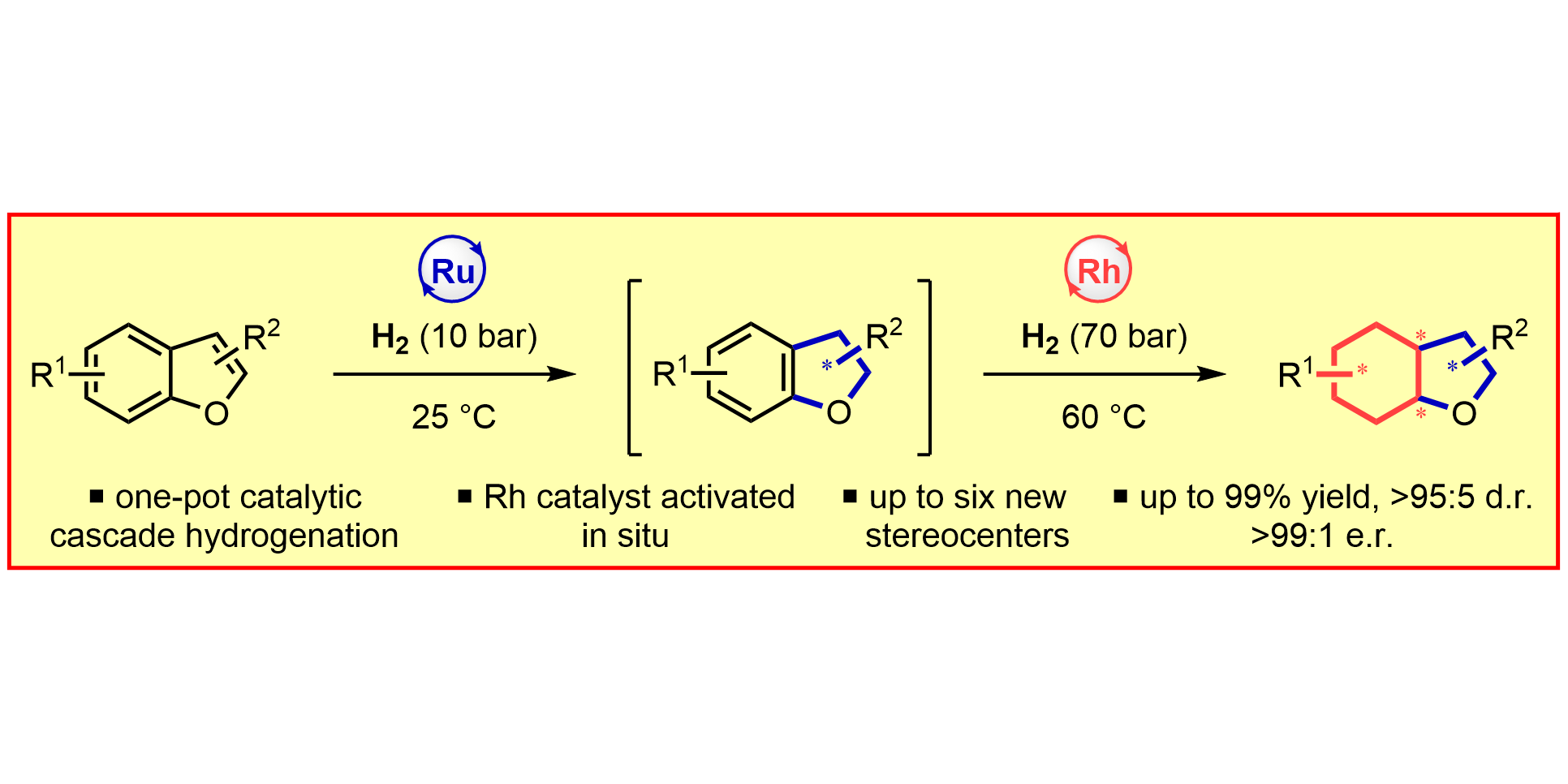
D. Moock, T. Wagener, T. Hu, T. Gallagher, F. Glorius,
Enantio- and Diastereoselective, Complete Hydrogenation of Benzofurans by Cascade Catalysis,
Angew. Chem. Int. Ed. 2021, 60, 13677-13681; Angew. Chem. 2021, 133, 13791-13796.
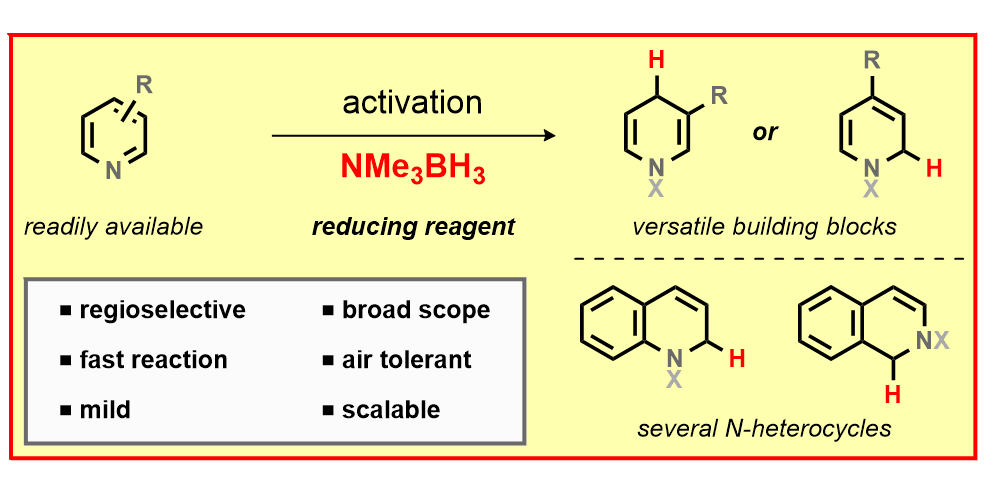
A. Heusler,§ J. Fliege,§ T. Wagener, F. Glorius,
Substituted Dihydropyridine Synthesis by Dearomatization of Pyridines,
Angew. Chem. Int. Ed. 2021, 60, 13793-13797; Angew. Chem. 2021, 133, 13912-13916.
§ These authors contributed equally.
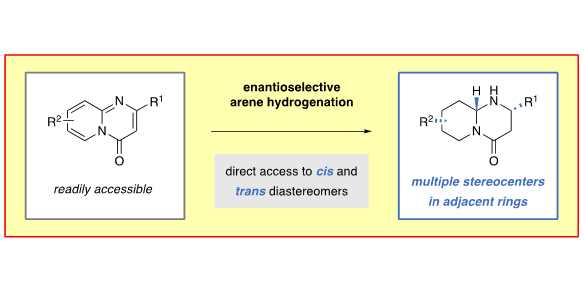
M. P. Wiesenfeldt,§ D. Moock,§ D. Paul,§ F. Glorius,
Enantioselective Hydrogenation of Annulated Arenes: Controlled Formation of Multiple Stereocenters in Adjacent Rings,
Chem. Sci. 2021, 12, 5611-5615.
§ These authors contributed equally.
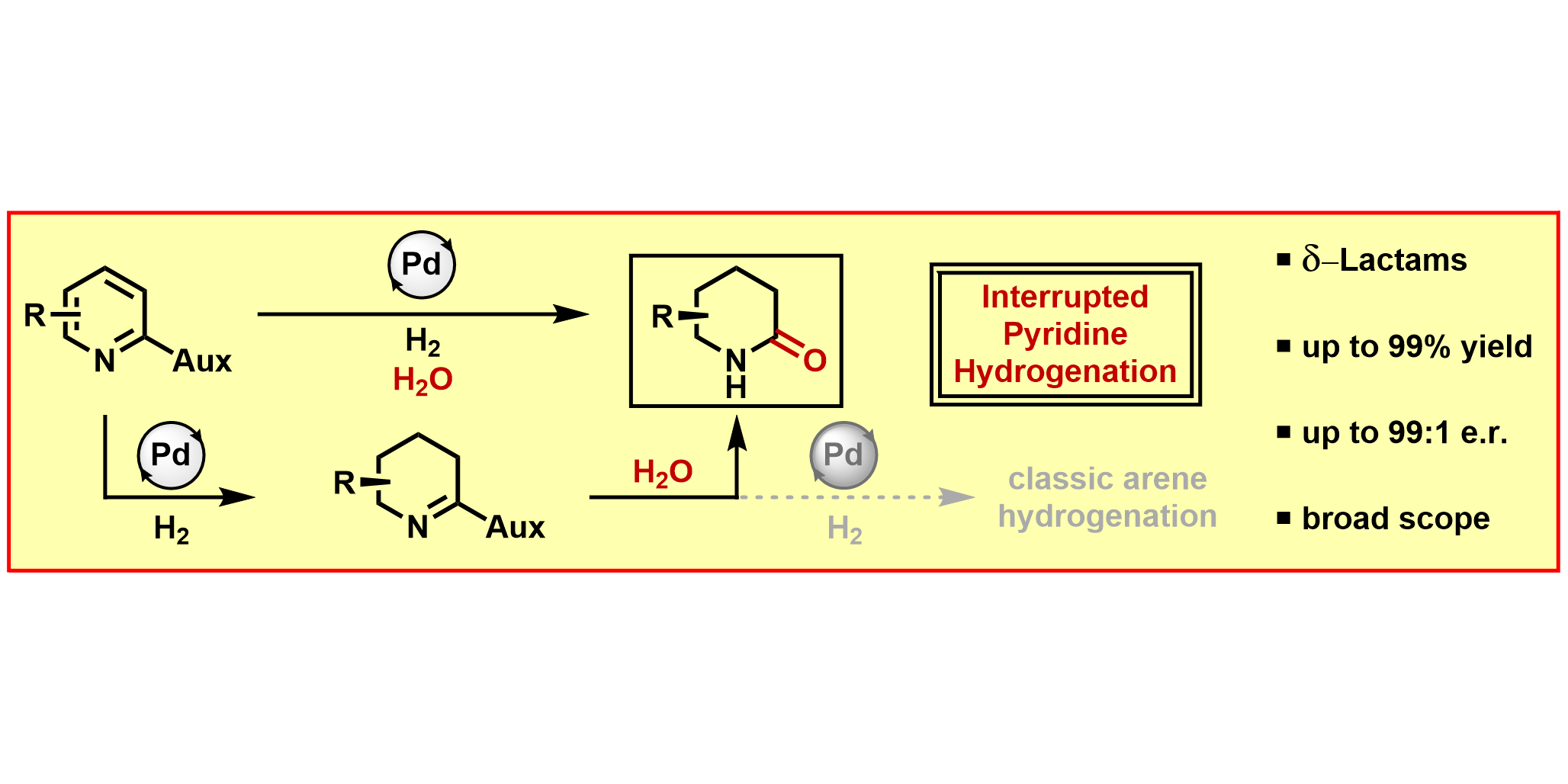
T. Wagener,§ L. Lückemeier,§ C. G. Daniliuc, F. Glorius,
Interrupted Pyridine Hydrogenation: Asymmetric Synthesis of δ-Lactams,
Angew. Chem. Int. Ed. 2021, 60, 6425-6429; Angew. Chem. 2021, 133, 6496-6500.
§ These authors contributed equally.
T. Wagener, A. Heusler, Z. Nairoukh, K. Bergander, C. G. Daniliuc, F. Glorius,
Accessing (Multi)Fluorinated Piperidines Using Heterogeneous Hydrogenation,
ACS Catal. 2020, 10, 12052-12057.
M. Wollenburg, A. Heusler, K. Bergander, F. Glorius,
trans-Selective and Switchable Arene Hydrogenation of Phenol Derivatives,
ACS Catal. 2020, 10, 11365-11370.
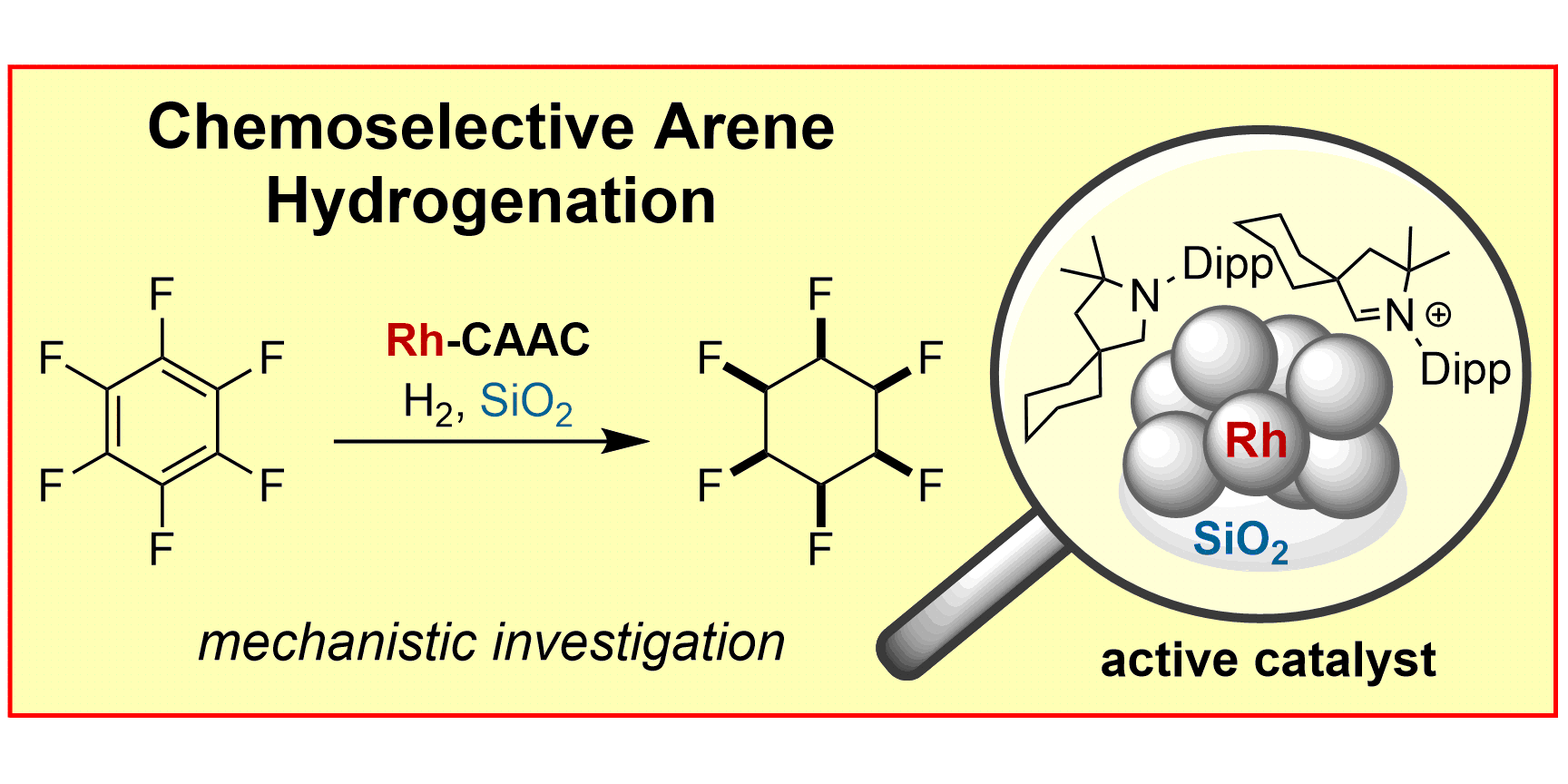
D. Moock, M. P. Wiesenfeldt, M. Freitag, S. Muratsugu, S. Ikemoto, R. Knitsch, J. Schneidewind, W. Baumann, A. H. Schäfer, A. Timmer, M. Tada, M. R. Hansen, F. Glorius,
Mechanistic Understanding of the Heterogeneous, Rhodium-Cyclic (Alkyl)(Amino)Carbene-Catalyzed (Fluoro-)Arene Hydrogenation,
ACS Catal. 2020, 10, 6309-6317.
W. Li,* T. Wagener, L. Hellmann, C. G. Daniliuc, C. Mück-Lichtenfeld, J. Neugebauer,* F. Glorius,*
Design of Ru(II)-NHC-Diamine Precatalysts Directed by Ligand Cooperation: Applications and Mechanistic Investigations for Asymmetric Hydrogenation,
J. Am. Chem. Soc. 2020, 142, 7100-7107.
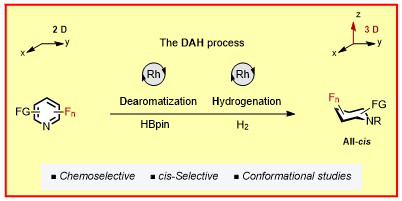
Z. Nairoukh , M. Wollenburg, C. Schlepphorst, K. Bergander, F. Glorius,
The formation of all-cis-(multi)fluorinated piperidines by a dearomatization–hydrogenation process,
Nature Chem. 2019, 11, 264-270.
M. Wollenburg,§ D. Moock,§ F. Glorius,
Hydrogenation of Borylated Arenes,
Angew. Chem. Int. Ed. 2019, 58, 6549-6553; Angew. Chem. 2019, 131, 6621-6625.
§ Both authors contributed equally.
M. P. Wiesenfeldt, T. Knecht, C. Schlepphorst, F. Glorius,
Silylarene hydrogenation - a strategic approach enabling direct access to versatile silylated saturated carbo- and heterocycles,
Angew. Chem. Int. Ed. 2018, 57, 8297-8300; Angew. Chem. 2018, 130, 8429-8432.
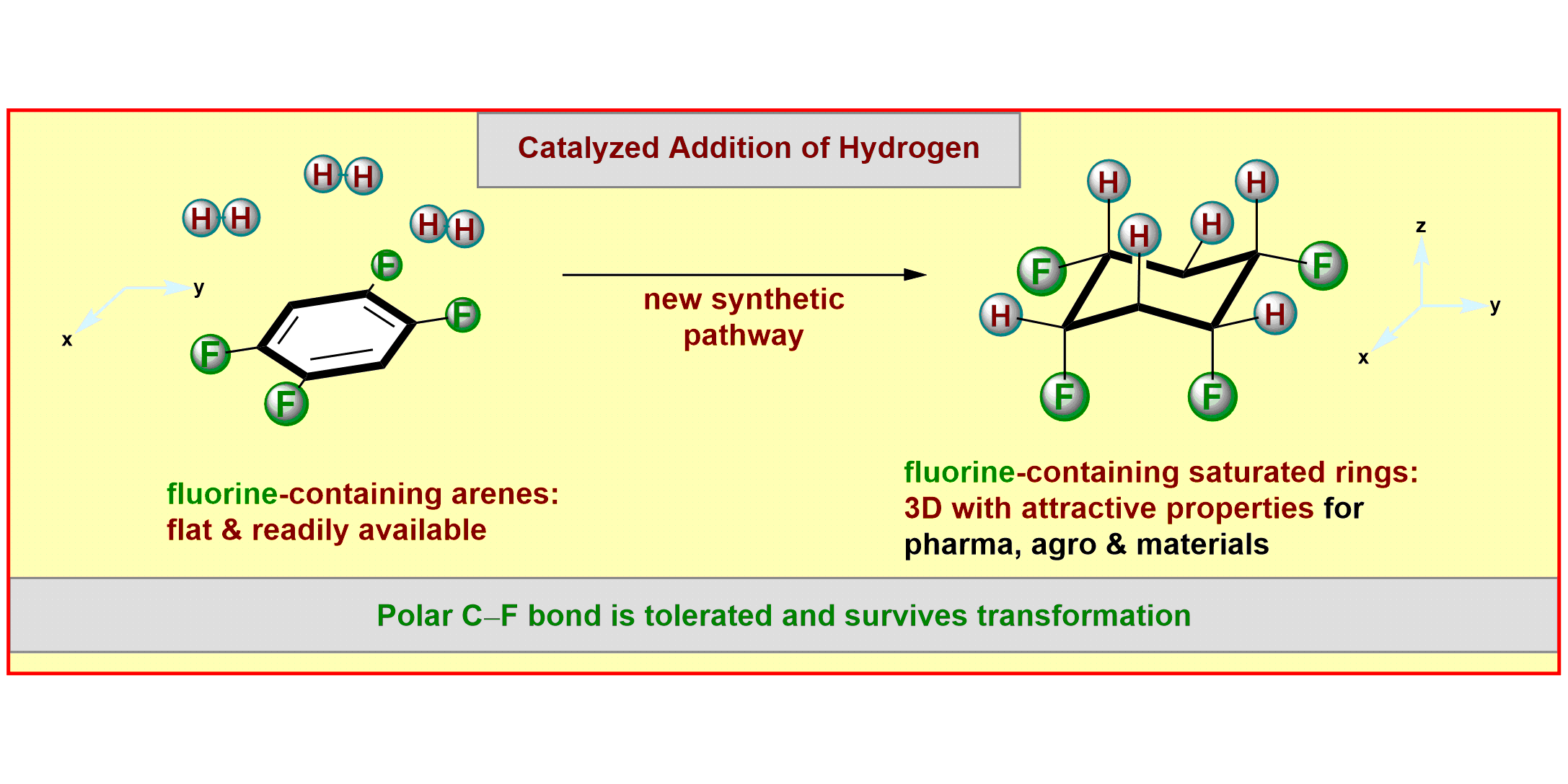
M. P. Wiesenfeldt, Z. Nairoukh, W. Li, F. Glorius,
Hydrogenation of fluoroarenes: Direct access to all-cis-(multi)fluorinated cycloalkanes,
Science 2017, 357, 908-912.
D. Janssen-Müller, C. Schlepphorst, F. Glorius,
Privileged Chiral N-Heterocyclic Carbene Ligands for Asymmetric Transition-Metal Catalysis,
Chem. Soc. Rev. 2017, 46, 4845-4854.
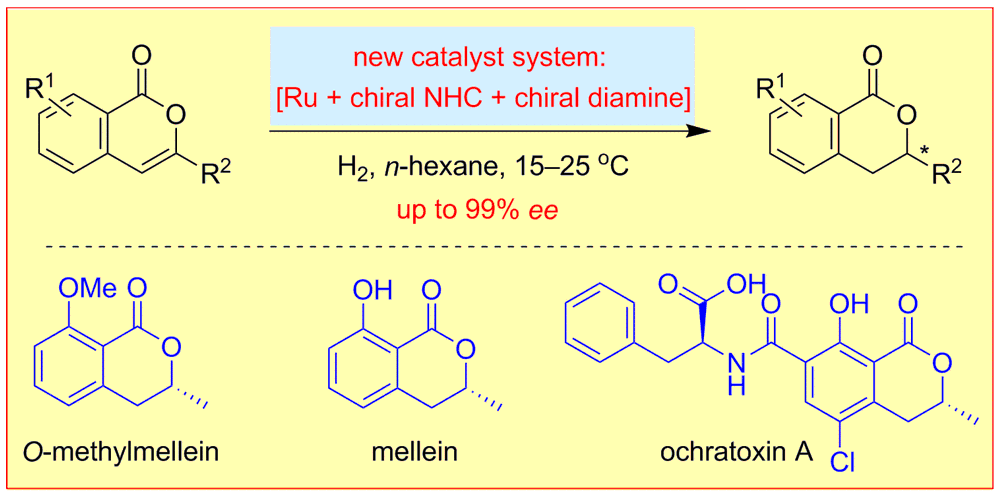
W. Li, M. Wiesenfeldt, F. Glorius,
Ruthenium−NHC−Diamine Catalyzed Enantioselective Hydrogenation of Isocoumarins,
J. Am. Chem. Soc. 2017, 139, 2585-2588.
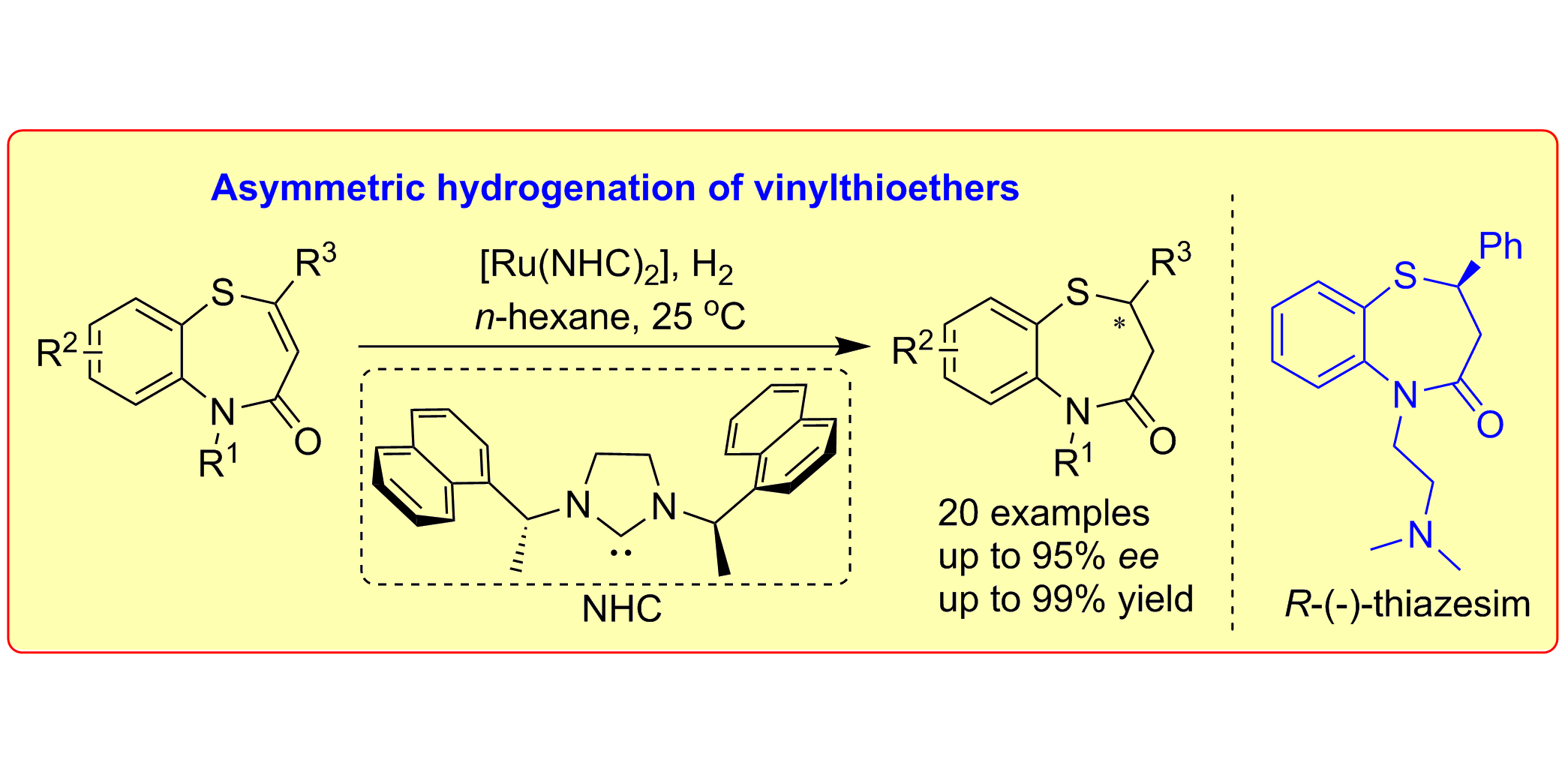
W. Li, C. Schlepphorst, C. Daniliuc, F. Glorius,
Asymmetric Hydrogenation of Vinylthioethers: Access to Optically Active 1,5-Benzothiazepine Derivatives,
Angew. Chem. Int. Ed. 2016, 55, 3300-3303; Angew. Chem. 2016, 128, 3361-3364.
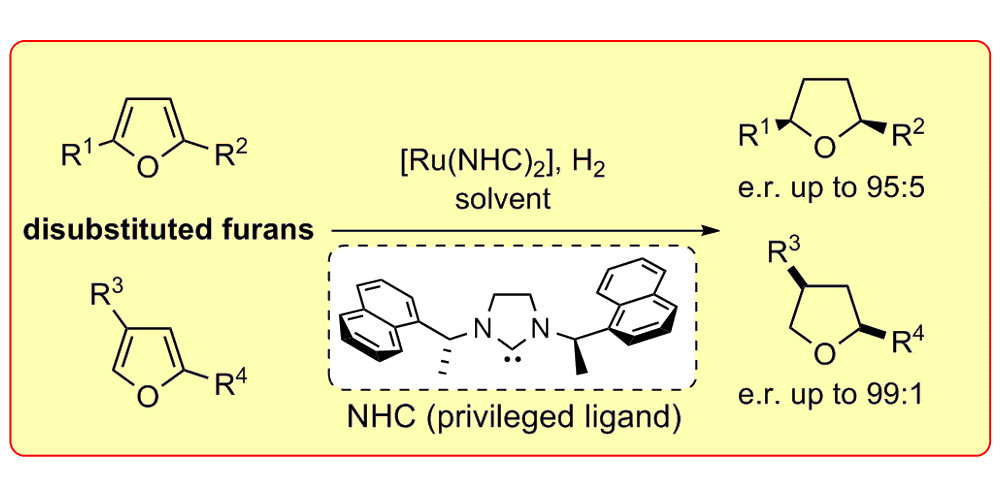
J. Wysocki, N. Ortega, F. Glorius,
Asymmetric Hydrogenation of Disubstituted Furans,
Angew. Chem. Int. Ed. 2014, 53, 8751-8755; Angew. Chem. 2014, 126, 8896-8900.
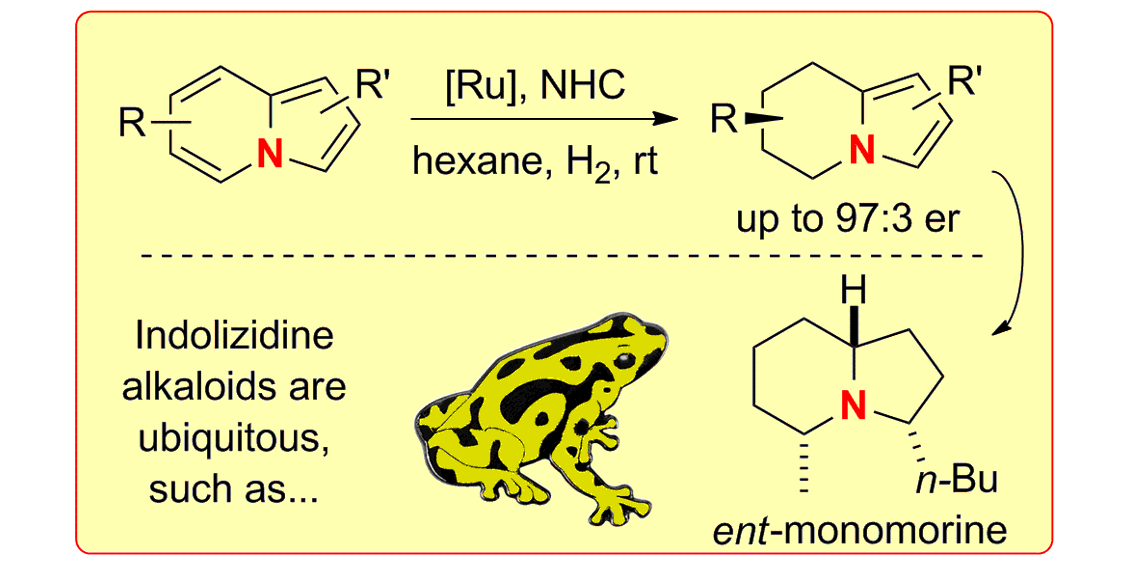
N. Ortega, D.-T. D. Tang, S. Urban, D. Zhao, F. Glorius,
Ruthenium-NHC Catalyzed Asymmetric Hydrogenation of Indolizines: Access to Indolizidine Alkaloids,
Angew. Chem. Int. Ed. 2013, 52, 9500-9503; Angew. Chem. 2013, 125, 9678-9681.
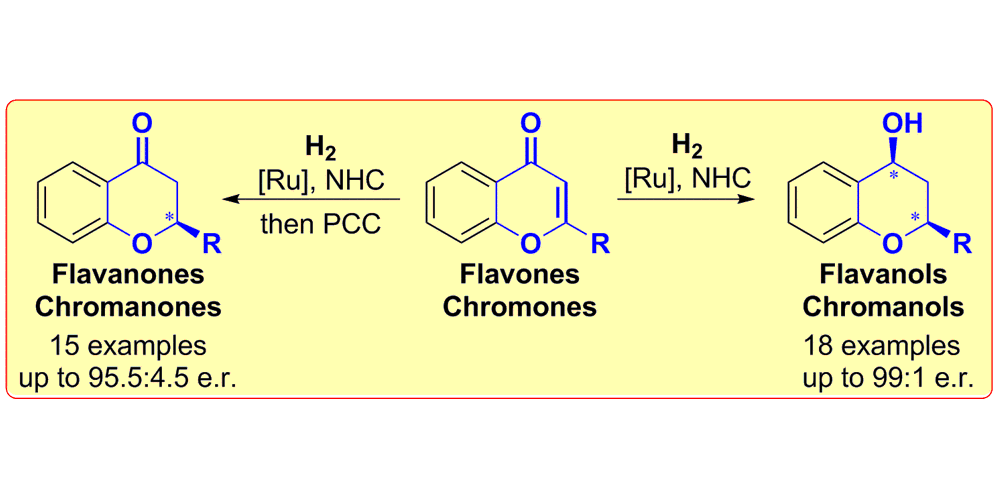
D. Zhao, B. Beiring, F. Glorius,
Ruthenium-NHC Catalyzed Asymmetric Hydrogenation of Flavones and Chromones: General Access to Enantio-enriched Flavanones, Flavanols, Chromanones and Chromanols,
Angew. Chem. Int. Ed. 2013, 52, 8454-8458; Angew. Chem. 2013, 125, 8612-8616.