Mass Spec Terms
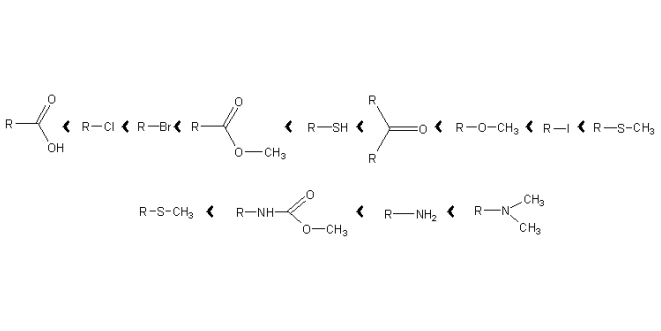
α-Cleavage – The homolytic fragmentation is induced by a radical cation located at a hetero atom. Functional groups can be arranged by their ability to induce α-Cleavages.
Allyl cleavage – Homolytic fragmentation of an allyl bond. This reaction is energized by the formation of a stabilized cation. Similar ==> benzyl cleavage.
amu - atomic mass unit, a historic unit for atomic weight - it is based on the 1/16th part of the 16O mass. Used in discussions, should not be used in publications.
Basepeak – this is the most intense ion within the measured (or regarded) mass range. In diagrams it is often set to 100% and the other ions are normalized to this intensity.
Benzyl cleavage – Homolytic cleavage induced by the formation of a cation stabilized by an aromatic system. The term is also used if the aromatic system is not a phenyl.
Bieman shift – If you alter the mass of a molecule by a chemical reaction and this new compound follows the same fragmentation scheme than the original, you can identify the site of alteration by the mass shift of the appropriate fragment(s). This is a trivial rule but helpful. If you are looking for derivatives which will not alter the fragmentation think of Deuterium or 13C instead of 1H or 12C.
Competition for the charge – During a fragmentation both parts compete for the charge. Usually the charge remains on the moiety with the lower ionization potential.
Concept of localized charge – To explain fragmentation schemes of radical cations (EI-ionization) it is often useful to argue as if the charge and the radical function are localized.
Dalton – Mass unit based on the 12th part of the mass of the isotope 12C. The abbreviation is u but Da ist also used. 1/1000th part ist abbreviated as mu or mmu.
Daughter ion ==> product ion
double bond equivalent (DBE) – The maximum number of hydrogens of a compound can be calculated by Hmax = (number of C-atomes) x 2 + (number of N-atomes) + 2. The DBE number is the difference of Hmax – Hfound devided by two. The DBE gives the total number of double bonds and rings.
Double bond location – The position and e/z nature of a double bond is often not reflected in the EI-mass spectrum. There are several possibilities of derivatization (bishydroxylation, formation of di(thiomethyl)ethers etc. The double bond positions in fatty acids can be elegantly determined by the ESI-MS/MS-fragmentation of the lithiated lithiumsalt.
Doubly charged ions – Mass separation depends on the m/z ratio of the ion. An ion with a charge of 2 appears at „half mass“. The charge state can be deduced from the spacing of isotope peaks in a cluster. With singly charged ions the mass difference is 1Da with doubly charged ½ etc. In EI-spectra one can expect mainly single charged ions, double charged ions occur with highly unsaturated compounds.
Electrospray ionization (ESI) – This is the softest ionization method. It uses the transfer from ions which are preformed in solution into the gas phase. In reality it is not an ionization method (because the ions are already present in solution), therefore it is often refered to as electrospray (ES). There is a chance to detect clusters of molecules hold together by hydrogen bonds.
Exact mass determination, accurate mass determination – Determination of the mass peak to a precision of (or better than) ±5 mmu. This precision often allows the user to assign an elemental composition to this peak. This measurement gives no decision about the purity of a compound, it is only characteristic for this peak. If there are more than one signal at the same nominal mass, then it is necessary to increase the resolution. With ==> high resolution they can be measured indipendently.
Excess energy – When a molecule is ionized by electron impact, approximately 2-10eV enegy is deposited on the molecule additionally to the ionization energy. This leads to a vibrational exitation and to fragmentation. The time until the ions leave the source region (with sector field instruments) is about 10-8s.
Fast atom bombardment FAB – The compound is mixed with a liquid matrix like glycerol. Then this mixture is put on a metal surface with is brought into the ion source by a push rod through a vacuum lock. Ions are produced by bombarding this target with fast neutral molecules or ions (than the method is called ==> liquid SIMS). This method is set marginated by electrospray but some researchers like it because it produces structure relevant fragments. Mass spectroscopists call it a dirty method, because „huge“ amounts of half volatile matrix is introduced in the ion soure. This results in a signal on each mass.
Field desorption (FD) – Very soft ionization technique. The compound is deposited on a fine tungsten wire which is coated with carbon whiskers. Ions are released from this wire by means of a high electrical field. Because this method is experimentally quite delicate it is mostly replaced by ==> MALDI, ==> FAB, ==> ESI. It is still useful for the investigation of nonpolar labile compounds.
FWHM ==> Resolution
High resolution – if a mass spectrometer is adjusted to a resolution of > 5000 (m/Δm), than this is called high resolution. This is necessary to distinguish between ions with the same nominal (integer) mass but a different exact mass (due to different elemental compositions).
Ion cluster – This is a group of ions which belong together, all peaks have the same elemental composition but with different isotopes of the participating elements. The resulting pattern can be calculated (by computer programs, MassLynx, Isopro etc).
Iontrap – spheric – Mass analyzer – It is possible to store ions with an electrical ac-field in a space which is formed by a ring and two caps above and beneath the ring openings. By variation of the AC-parameters (frequency, amplitude and dc-offset) it is possible to influence the storage capabilties of such an ion trap. E.g. by increasing the amplitude ions are expelled and detected starting with the low m/z ratios. Iontraps are capable of doing MS/MS/.. experiments in a (time) sequential mode in the same volume.
Iontrap – linear– Mass analyzer – with this quadrupol device the ions are trapped beween potential barriers at the end. The mass scan is accomplished by destabilizing the ions.
Ion cyclotron resonance fourier transform mass spectrometer ICR-FT-MS – This is a mass analyzer where ions which are trapped in a homogenuous magnetic field are excited by an electrical pulse. The ions travel on circular paths. The cycle time depends on their m/z value (and the magnetic field strengh). These moving ions induce alternating electric potentials in detector plates. The resultion ac-voltage is the sum of all frequencies and can be analyzed by a fourier transformation.
Advantages:
- All ions in the cavity are used for measurement none are excluded by slits.
- The spectrometer is not scanned, all ions are used simultaneously.
- Ions are not destroyed by the measurement, so they can be used several times (multiplex advantage). By this sensitivity and/or resolution can be increased.
- Resolutions of more than 106 are easily obtained.
Ionization energy – this is the energy needed to convert neutral molecules to ions. To produce radical cations (EI) 6-11eV are needed.
Ion series – Ions with a constant mass difference, they are not necessarily formed through the same fragmentation path.
"Isotopic resolution" – This is an (colloquial) expression, that two masses which differ by 1 Da are separated (by 10%, 50% valley).
Key ion – A Fragment which gives a hint for a specific (sub)structure. E.g. the m/z 74 for α-unsubstituted methyl esters. Pay attention! Most "sure" fragmentations can be overridden by more favourable ones.
LDI -Laserdesorption/ionisation – Evaporation and ionization of a compound with a short intense laser pulse. The power densitiy needed for organic molecules is > 5 MW / cm2. Mass analysis is usually done by a TOF-MS.
Loss of neutrals – The formation of small stable neutrals is often the driving force for fragmentations. The favorable formation of H2O, HCN, CO, CO2, etc. often overrides enery barriers.
"low voltage spectrum" – This is an old method (EI-MS) to enhance the relative intensity of the molecular ion. When organic molecules are ionized with electrons just above their ionization level the excitation of the molecule by excess energy is minimized and by this fragmentations are suppressed. This method gives poor sensitivity. MW are better determined by CI, ESI or MALDI.
m/z - The three-character symbol m/z is used to denote the dimensionless quantity formed by dividing the mass of an ion by the unified atomic mass unit and also by its charge number (regardless of sign). The symbol is written in italicized lower case letters with no spaces.
MALDI Matrix Assisted Laser Desorption/Ionization – This is a variant of LDI. A compound is cocrystallized with a 50-1000fold excess of a matrix compound (e.g. 2,5-dihydroxybenzoic acid). By this the power density of the laser can be reduced by a factor of 10. Very efficient for high molecular weight compounds (peptides, proteins, RNA and DNA fragments).
Mass dependent rounding – It is convenient to use integer masses for discussion and calculation. For this the observed masses must be rounded by software. Most organic compounds contain hydrogens and because the mass of H is 1.0078, this increases the decimal places of the ion mass with increasing H-content. E.g. the monoisotopic mass of C50 H102 is 702.7981 and would be rounded to 703, desirable would be the nominal mass 702. Some MS-software uses a mass dependant rounding level to cover this effect and give "good" integer masses up to m/z 1000. Potentially dangerous!
McLafferty Rearrangement – This rearrangement has a photochemical equivalent called Norrish Type II. You may expect this fragmentation with many molecules containing double bonds, mainly esters, ketones, aromatics with aliphatic side chains etc. When there is a disubstituted double bond in an aliphatic chain, their isomerization is faster than the McL.
McLafferty + 13 (+27, +41 ...) – A rearrangement of ketones and esters which starts with a nonspecific shift of a H-atom from the C6-C10 region. After this a H-atom near the charge center is shifted back to this radical site (with the McLafferty+13 it originates from the α-position). Finally there is a bond fragmentation induced by this radical site between the β-π bond.
Mother ion ==> precursor ion
Nominal mass – This mass value is formed by the addition of rounded masses of the pure nuclides. This mass value makes only sense below m/z 500 because of the discrepancy of the NM with the real mass.
"Odd Electron Rule" – This rule declares that you can only loose a radical once in a fragmentation series. The reason ist, that a homolytic fragmentation usually need so much energy that the remaining rest is only sufficient for low energy rearrangements. This rule has (many) exceptions – but they have all an explanation (e.g. the easy formation of stabilized radicals with polyiodide compounds etc.).
Onium-fragmentation – This fragmentation often follows an α-Cleavage . It releases an olefin under rearrangement of a H-atom.
Orbitrap – A very recent development, where ions are mass-analysed by observing their cyclic motion between especially shaped electrodes. The information is gained by a Fourier transformation of the signal.
Ortho-effect – often the ortho pattern enables fragmentations which are not possible (distance!) with the meta or para isomers. By this the mass spectra of the m- and p-isomer are often very similar.
Precursor ion ("mother ion") – Ion which is the source of a fragmentation either spontaneous or induced by collisions.
Product ion – Ion which is produced by a fragmentation, it is often used with MS-MS-methods.
Pyrolytic fragmentation – often it is difficult to decide whether a fragmentation is induced by electron impact or by temperature. The choice of short GC-columns (with GC-MS) or "soft" ionization techniques is recommended.
Quadrupol mass spectrometer – symmetric array of four rods of which the opposite parts are electrically connected. A alternating voltage is applied to this system. When an ion travels through the axis of this array it is forced to a screwlike movement. The electrical conditions can be adusted that either the whole m/z range or only a small m/z range manage to pass through the system. This system acts as a mass filter.
Radical fragmentations – these often results in the fragmentation of only one bond. These reactions are fast but often need much energy. (see also "odd electron rule").
Real mass (exact mass) – This is the mass calculated from the masses of the pure nuclides (with the decimal places). If there is only one isotopic compostition on this mass it is called monoisotopic mass. This is the mass for exact mass determination.
Rearrangements – forming of new bonds as a consequence of the excitation. Hydrogens are most likely to move, but there are also other mobile substituents (silyl, phenyl, etc.). Sometimes very long distances are bridged by rearrangements.
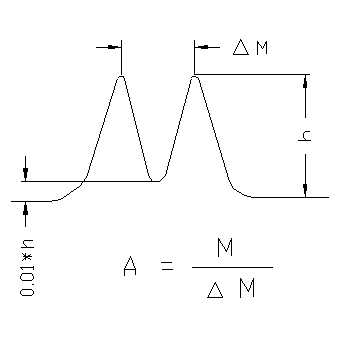
Resolution – Ability of a mass spectrometer to separate ions of different m/z values. This is an important qualitiy item of an instrument. There are different definitions:
- 10% valley-definition of resolution. If two peaks are seperated, so that they valley between them is 10% of the peak height, then the resolution is R=m/Δm. With sector field spectrometers this ratio is constant over the entire mass range. This is not the case with other types of analyzers.
- 50% valley-definition of resolution see 10% valley definition.
- Peakwidth definition m/Δm: For a single charged peak the ratio of the mass and the peakwidth at a defined height (e.g. 5%, 10% 50%). The 50% variant is often used for time of flight spectrometers and is called FWHM-definition (full width at half mass).
Retro-Diels-Alder-reaction – This is a common fragmentation type when the structural prerequisites are fulfilled.
Secondary ion multiplier SEM – The detector type is sensitive to fast moving particles (photons, ions, neutrals). When they hit the conversion dynode electrons are released. They are accellerated by an electrical field and hit another electrode where they release more electrons. This process is repeated several times, it is comparable to an avalanche. At the end of the cascade the impuls (or the current) is registrated. Amplifications of 104 to 107 are possible.
Sector field mass spectrometer – Mass analyzer with one (or more) magnetic and often additional electric field regions. The magnetic sector causes impulse-focussing, the electric field causes velocity-focussing properties. With special geometries all ions are focused in a plane, this makes planar detectors (photoplate etc) possible.
Secondary ion mass spectrometry – Ionization method where ions are sputtered out of a solid or liquid surface by fast primary ions (Cs+, Ar+, etc). The most interesting feature is the geometric resolution on the specimen. Images with μm resolution can be genarated.
Silylmigration – Silyl groups rearrange very easily (proton equivalent!), especially oxygen and halogen are very strong attracting groups.
Stoichiometric mass (equiv. formula mass) – This is the mass which is used by the synthetic chemist, it is calculated by the addition of the mean atomic weights of the elements with their natural isotopic distribution. Within a mass spectrum it is the center of gravity of an isotope cluster. When the isotop peaks are not resolved (e.g. in the high mass range), please use this value for calculation.
Total ion current – With GC-MS and HPLC-MS the chromatogram must be reconstructed through the spectrum information. Because the spectra are measured in short constant intervals (approx. 1s). The absolute intensity of all ions can be added and plotted versus the spectrum number (or time). This trace is called total ion current (TIC) or integrated intensity (II).
Thompson – (abbrev. Th) this is the mass value in Da devided by the numer of charges.
u – atomic mass unit, 1/12 mass of carbon isotope 12C. (1u = m(12C)/12 [kg]).
