Dynamic cell contacts
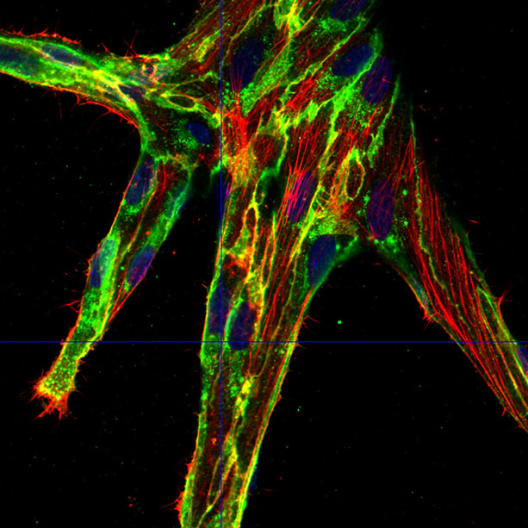
During angiogenesis, blood vessels are formed from vessels that already exist, for example when an organism develops or when wounds heal. Vessels can likewise sprout during some diseases – but in such cases they are misregulated, for example when tumours form. The interior wall of a blood vessel consists of endothelial cells. If a new network of vessels develops, these cells move forward and rearrange themselves. They also have to form new contacts with other neighbouring cells. But how does that happen? And how, ultimately, do the cells move forwards in directed manners? Researchers at the Cells-in-Motion (CiM) Cluster of Excellence at the University of Münster have decoded a series of molecular mechanisms that are behind this. They present a model in which the extension of endothelial cells becomes highly important during angiogenesis. The cell contact protein VE-cadherin, which occurs on the surface of endothelial cells, also has an important function. “Our findings provide a solid basis for a model that can explain the mechanisms of the cytoskeleton and VE-cadherin dynamics at the subcellular level in angiogenesis,” says lead author Dr. Jiahui Cao. The study has been published in the current issue of “Nature Communications”.
The detailed story:
The VEGF protein is responsible for the formation of new blood vessels. It functions as a signalling substance, and influences for example the migration of new endothelial cells into a wound. In order to observe how endothelial cells and their contacts interact during angiogenesis, the researchers studied retinas permeated by blood vessels in developing mice, and they also looked at cultures from endothelial cells which they had taken from veins in human umbilical cords and exposed to the VEGF signalling substance. They observed the dynamics of the cell contacts using a variety of light microscopic techniques.
For this purpose, the researchers – under CiM team leader Prof. Dr. Hans-Joachim Schnittler – first stained the VE-cadherin protein on the endothelial cells with fluorescent dyes. This protein acts as a kind of zipper between them, engaging in close interplay with the cytoskeleton – and especially with one of its components, actin. The researchers had already discovered in an earlier study that if a cell increases its circumference, for example in growing cells, this results in a lower concentration of VE-cadherin at the cell contacts. This in turn results in protrusions consisting of actin forming at the cell contacts, causing the contacts to change dynamically. These cell protrusions are called JAIL, which stands for ‘junction-associated intermittent lamellipodia’.

The researchers assumed that the mechanism also plays a role during angiogenesis. They observed that endothelial cells which increased in length induced by VEGF displayed a greater circumference and, at the same time, longer cell contacts. The quantity of the VE-cadherin protein on the surface of an elongated endothelial cell did not increase, but stayed constant – indeed less VE-cadherin occurred at the individual cell contacts than before due to increase in perimeter. The researchers discovered that, at the junctions where there was reduced VE-cadherin, JAIL formed which overlapped with other cells and, in turn, gave rise to new adhesion sites for VE-cadherin. In addition, the researchers proved that the protein complex Arp 2/3 has to be active for the formation of JAIL. This protein complex plays an important role in the formation of actin-driven protrusions and is itself regulated by certain proteins.
Interestingly, the researchers discovered these dynamic changes in the cell contacts primarily from the positions of the cells – aligned to the direction of their migration. A large JAIL was formed in the leading front allowing the cell to migrate towards its destination. In the process, new VE-cadherin adhesion sites acted as anchors holding fast to their environment. At the same time, small JAIL were formed on the side of the cell, and a neighbouring cell which was attached to them was able to use the JAIL to move forward itself. The researchers identified two more factors which were important for this – for one thing, the Rac protein, which was already known for being responsible for the formation of protrusions at cell contacts. The researchers were able to demonstrate that if they inhibited the protein, no JAIL were formed.
At the same time, the researchers discovered a loss of tension in the actin and myosin fibres at the cell contacts, as a result of which the cells were able to become elongated. It was a series of consecutive signals which triggered the loss of tension. The VEGF protein bound an enzyme which itself triggered activity in a further enzyme, myosin light-chain kinase II. The researchers also observed that microtubules, likewise filaments of the cell cytoskeleton, rearranged themselves at the cell contacts – which also contributed to the loss of tension and to higher dynamics at the cell contacts.
The results might be relevant for research into diseases – for example, to develop medicines later which influence these molecular mechanisms. Whether the results of this basic research might be reflected in potential applications cannot be predicted at the present time. In future, the researchers first want to continue their investigations into the individual signalling mechanisms in order to learn more about how they activate and inhibit one another.
Collaboration between various groups of researchers
A number of different teams at the Cluster of Excellence worked on the study. The CiM team of researchers led by developmental biologist Prof. Ralf Adams is specialized in studying angiogenesis in the retinas of mice, and carried out research into the occurrence of VE-cadherin in genetically modified animals. The research team headed by Prof. Erez Raz investigated the activity of the Rac protein using a special technique – fluorescence resonance energy transfer. Furthermore they got support from a research group from Prague who work on artificial intelligence. “Collaboration with the other teams of researchers was extremely open,” says physician Prof. Hans-Joachim Schnittler, “and discussion of the data and their interpretation were very purposeful.”
The study received funding from the Cells-in-Motion Cluster of Excellence at the University of Münster, the German Research Foundation, the Federal Ministry of Education and Research and the Czech Science Foundation.
Original publication:
Cao J, Ehling M, März S, Seebach J, Tarbashevich K, Sixta T, Pitulescu ME, Werner A-C, Flach B, Montanez E, Raz E, Adams RH, Schnittler H. Polarized actin and VE-cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis. Nat Commun 2017; 8; DOI: 10.1038/s41467-017-02373-8. Abstract

