Making digital 3D images of tissue
When researchers and physicians analyse tissue, for example in order to investigate any pathological changes, they often look at the tissue samples under the light microscope. However, producing meaningful images is not always easy. Researchers at the Cells-in-Motion Cluster of Excellence at the University of Münster and at the Max Planck Institute for Molecular Biomedicine in Münster have now developed a new method which, in the case of lymphoedema, can create digital 3D images of blood vessels and lymphatic vessels of entire tissue biopsies. This method will help to analyse the underlying changes of the blood and lymphatic vessels in lymphoedema in a more detailed way. “We’re doing a digital three-dimensional histopathology,” explains Dr. René Hägerling, lead author of the study which has just been published in the latest issue of the “JCI Insight” journal.
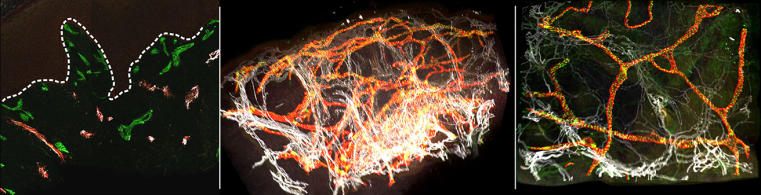
The process has involved interdisciplinary collaboration between biochemists, chemists, computer scientists, biologists and physicians. The researchers analysed three skin biopsies taken from healthy persons and one skin biopsy of a patient with lymphoedema. Using light sheet microscopy they produced thousands of individual optical sections for each sample. Using a special programming system called Voreen the researchers assembled the individual optical sections on the computer and produced a three-dimensional reconstruction of the tissue structure. The new method – called VIPAR – enables researchers for the first time to generate 3D reconstructions of skin biopsies, visualize them and extract characteristic parameters of the tissue. This method differs from traditional histological analyses, in which a tissue sample is sliced into many sections and each individual section is observed two-dimensionally. Whether the new technique can be used in clinical practice soon, cannot be forecast at the moment.
Original publication:
Hägerling R, Drees D, Scherzinger A, Dierkes C, Martin-Almedina S, Butz S, Gordon K, Schäfers M, Hinrichs K, Ostergaard P, Vestweber D, Goerge T, Mansour S, Jiang X, Mortimer PS, Kiefer F. VIPAR, a quantitative approach to 3D histopathology applied to lymphatic malformations. JCI Insight 2017;2, DOI 10.1172/jci.insight.93424. Abstract
The detailed story:
Lymphatic vessels transport tissue fluid – lymph – from the interstitial space between cells back into the blood circulation. If the transport process does not function properly, this can result in the formation of lymphoedema, a fluid accumulation in the skin. Lymphoedema can have a variety of causes – for example, injuries or operations, e.g. within the scope of breast cancer therapy. Lymphoedema can also be caused by genetic defects, which often manifest already at birth. Physicians do not have any well-developed and detailed imaging technique for the 3D visualization and diagnosis of the underlying vascular changes in lymphoedema. This lack of technique for investigation and diagnosis has been a major contributing factor to the limited knowledge about the development of lymphoedema and to a failure to develop effective drug and surgical therapies.
To overcome these limitations, research teams at the Cells-in-Motion Cluster of Excellence (CiM) have now jointly developed and tested a new method. The CiM team headed by Prof. Friedemann Kiefer is specialized in the modern microscopic analysis of tissue, in particular that of the lymphatic vessel system. Dr. René Hägerling and his colleagues first analysed three skin bioposies taken from healthy individuals. The researchers marked the walls of the lymphatic and blood vessels with fluorescent antibodies and then analysed the samples using the light sheet microscope. This microscope images each sample, layer by layer, using a light sheet generated by a laser beam, and a camera records the fluorescent signal. This generates data from several thousand individual optical sections for each sample.
Computer scientists in the CiM team led by Prof. Xiaoyi Jiang assembled the individual layers on the computer, using a special programming system called Voreen, which allowed a three-dimensional reconstruction of the entire tissue architecture. Using the new method, the researchers produced a detailed spatial visualization of the blood and lymphatic vessels, thus enabling them to segment the generated images. This meant they were able to obtain data on the most relevant tissue parameters. What is the size and form of the vessels in healthy tissue? In what patterns are vessels interconnected and how do they branch? From these parameters they were able to create algorithms for these typical patterns.
In a further step, the researchers analysed the skin biopsy of a patient with lymphoedema. The three-dimensional images showed marked changes in the vessel structure in comparison with the healthy skin samples. The most striking aspect was the fragmentation of lymphatic vessels which was not immediately identifiable using classical standard 2D histology. The data analysis also showed marked changes in comparison with healthy tissue.
The researchers call the new method for analysing tissue samples “VIPAR” – an abbreviation of “volume information-based histopathological analysis by 3D reconstruction and data extraction”. They plan to carry out further tests on the method and hope that in future it can help in better understanding lymphoedema diseases and in making more detailed diagnoses. Based on this, new therapeutic approaches could be developed. The researchers plan to perform first pilot studies with chosen patient-cohorts. René Hägerling is convinced: “This is a very promising method which can probably also be used in other organs too.”
The pilot study received funding from the Cells-in-Motion Cluster of Excellence at the University of Münster, the Max-Planck Institute of Molecular Biomedicine in Münster and the Collaborative Research Centre 656 (“Molecular Cardiovascular Imaging”) at Münster University.

