Fluorinated sugar molecules as vaccine leads against meningitis B and C
Meningococci (Neisseria meningitidis) are disease-causing bacteria that cause life-threatening meningitis, or blood poisoning (septicaemia). There are a number of meningococcal variants worldwide, known as serogroups, and in Germany the Standing Committee on Vaccination (STIKO) recommends immunisation against serogroups B and C in children. A team from the University of Münster (led by Prof. Dr Ryan Gilmour) and the Max Planck Institute (MPI) of Colloids and Interfaces in Potsdam (led by Prof. Dr Peter H. Seeberger) has now developed a combined vaccine lead from synthetic fluorinated sugar molecules that is effective against both strains simultaneously. The vaccine lead is the component of the vaccine that triggers the desired immune response. The study has been published in the "Journal of the American Chemical Society".
The introduction of fluorine into the natural structure brings many advantages, which begin with assisting in the synthesis and analysis, and enhancing metabolic stability. In this proof of concept study, the scientists demonstrated the efficacy of the lead compound in cell culture tests and in mice.
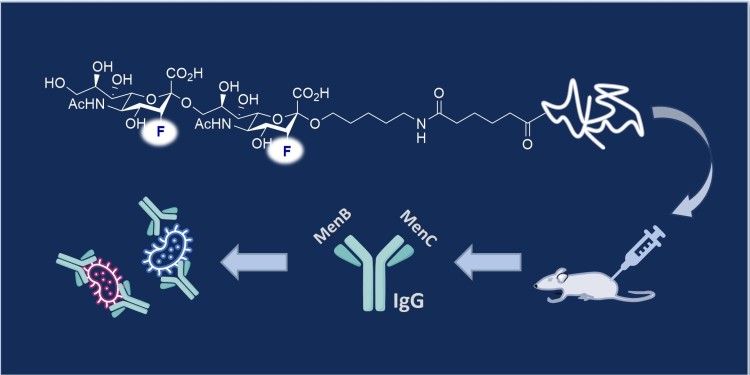
To produce the vaccine lead, the team mimicked the molecular fingerprint of the bacterial capsular polysaccharide (CPS) epitope. These polysaccharides form a shell around the bacteria, and their unique structures means that they function as antigens and can be specifically targeted by the immune response. This highly specific form of molecular recognition is the basis of a vaccine. The team synthesised a molecular structure that is closely similar to the epitope on the surface of the natural polysaccharide capsule and combined it with a carrier protein. Further investigations showed that this epitope analogue can trigger an immune response against serogroups B and C in mice.
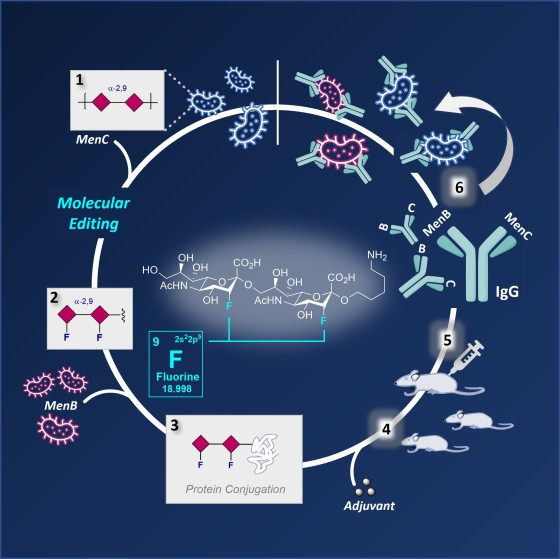
PhD student Patricia Priegue (MPI) combined the fluorinated epitope inspired by serogroup C (MenC) with an outer cell membrane protein of serogroup B (MenB) to enable the production of a bivalent vaccine. The newly designed molecule triggered the production of highly specific immunoglobulin G (IgG) antibodies, subtype IgG1. "The antibodies generated by this strategy protected against meningococci B and C. We have validated this in vitro by SBA (serum bactericidal antibody) and OPKA assays (opsonophagocytic killing assay), which indicates that these fluorinated glycoconjugates are promising vaccine leads," emphasises Peter H. Seeberger.
Funding
The European Research Council (ERC Consolidator Grant 818949) and the Max Planck Society provided financial support for the work.
Original publication
C. Jordan, K. Siebold, P. Priegue, P. H. Seeberger and R. Gilmour (2024): A Fluorinated Sialic Acid Vaccine Lead Against Meningitis B and C. J. Am. Chem. Soc. 2024; DOI: doi.org/10.1021/jacs.4c03179
