

Radical Chemistry in Organic Synthesis – Method Development
In organic synthesis selective bond forming reactions via C-centered radicals have become valuable tools for construction of complex systems. Carboaminoxylation, base promoted homolytic aromatic substitution and novel heteroatom radical precursors improve the general applicability of radical chemistry in organic synthesis. The Studer group is interested in the development of efficient oxidative C-C and C-X bond forming reactions by using nitroxides as mild oxidants. These methods allow for stereoselective vicinal bisfunctionalization of unactivated alkenes.
Read more: Radical Chemistry in Organic Synthesis – Method Development
Radical Carbaminoxylation and Beyond
With TEMPO being one of the key compounds in the Studer lab, early synthetic applications of alkoxyamines in organic synthesis focused on radical cyclization reactions using nitroxides, so called carboaminoxylation reactions.[19],[38],[40],[48] Early on, the potential of the persistent radical effect (PRE) was recognized[26],[43] for conducting selective radical chemistry. This research has further evolved towards various carbo-element functionalization reactions.[196]
Base Promoted Homolytic Aromatic Substitution,
Oxidations, Single Electron Transfer & Heterocycle SynthesisAlong with approaches to establish concepts of redox catalysis via electron transfer, the group has intensively been working on base promoted homolytic aromatic substitution reactions (BHAS). Mechanistic studies[179] paved the way to the application of the BHAS in cascade reactions. Such processes with aryl radicals[160] or more complex imidoyl radicals generated from isonitriles have been used for the synthesis of phosphorylated[186] or aroylated[180] phenanthridines and phenanthrenes[209]. Radical chemistry is also applied to prepare pharmaceutically important fluoroorganic compounds.[151],[175],[188],[199]
The Studer group has considerably contributed to the development of organic oxidation processes,[117] mediated or catalyzed by nitroxides[154],[174] and oxammonium salts,[136] where dioxgen is being used as the terminal oxidant in some cases. Aerobic cross-dehydrogenative coupling allows for preparation of pharmaceutical important isoxazoles.[125]
Nitrosoarenes have been extensively studied and exploited for the (stereoselective) synthesis of nitrogen containing organic compounds. Heterocylces are formed by formal addition of nitrosoarenes to donor/acceptor cyclopropanes ([3+2] cycloaddition[193]) or to allyltin derivatives (formal [3+2] cycloaddition[134]) to give substituted isoxazolidines. Nitrosoarenes react with arynes to form carbazoles ([2+2] cycloaddition[159]). The nitroso Diels-Alder reaction[73] has also been applied to natural product synthesis.[81],[98]New Methods for Radical Alkene Functionalization and for Generation of Radicals
Vicinal alkene bisfunctionalization by using commercially available radical precursors is studied. The Togni reagent has been used for the trifluoromethylaminoxylation of alkenes (introduction of -CF3 & -OR entity). [150] Even simpler reagents enable the aryloxidation with TEMPONa and diazonium salts[153] or with arylboronic acids and dioxygen.[111] Along these lines valuable reagents have been developed and applied to aminoazidation[190] and azidooxygenation[172] reactions. As a part of studies on new reagents for radical organic transformations, the Studer laboratory also found that catecholboron enolates serve as enoyl radical precursors when reacted with TEMPO.[97] Enones can be oxidized to α-aminoxylated β-chloroketones with chlorocatecholborane.[152]
C-C and C-X Coupling: Radical Chemistry Complements Transition Metal Mediated Processes
Oxidative C-C bond forming reactions have been successfully conducted with Grignard reagents in the presence of the TEMPO radical to give the corresponding homocoupled products,[89],[96],[110] allowing for a novel entry towards the synthesis of conjugated polyaromatic polymers.[106] The concept was later extended towards the coupling of functionalized moieties such as nitrones.[135] All these transformations[149] are complementary to transition metal mediated processes. The homocoupling reaction can also be used as an initiation step to perform the conceptually new radical/anionic SRN1-type polymerization of dihaloarenes.[155]
The Studer group has also developed novel methods for the introduction of phosphorous entities and amino groups. Tin and silicon based precursors allow the modular synthesis of phosphanes,[72],[124] including the synthesis of chiral ligands[107] and building blocks for π-conjugated systems with potential importance in optoelectronics.[137] Radical N-alkylation[104] was achieved by an analogous protocol. In this context, also radical transfer hydroamination reactions[69],[75] using polarity reversal catalysis[121] and direct amidations of (hetero)arenes using photoredox catalysis[207] have been developed.
Radicals and Catalysis
As compared to non-radical processes, most radical reactions are fast. Radical reactions require only a small amount of initiator, addition of a catalyst is generally not necessary. The Studer group is working on catalytic processes, where radicals or radical ions occur as intermediates. The group has pioneered the field of oxidative carbene catalysis, where acyl azolium ions as intermediates allow for highly stereoselective cascade reactions. Novel strategies for transition metal catalyzed processes using radicals as oxidants have been established. Several observations have shown that radicals are involved in a series of transition metal mediated transformations. The novel concept of the electron as an efficient catalyst for conducting various types of radical cascade reactions is currently explored.
Read more: Radicals and Catalysis
Mimicking Nature's Concepts – Oxidative N-Heterocyclic Carbene Catalysis
Fundamental insights into the mechanisms[147] of oxidative NHC catalyzed transformations involving the Breslow intermediate have been provided by the Studer laboratory[162] and various applications of oxidative NHC catalysis have been disclosed. Biomimetic organocatalytic oxidations of e.g. aldehydes proceed with the TEMPO radical as a mild organic oxidant in the presence of N-heterocyclic carbenes (NHCs) as efficient catalysts for these electron-transfer processes.[87] Oxidative NHC catalysis with aromatic and also aliphatic[198] aldehydes as substrates allows for selective acylation of alcohols in the presence of amines.[100],[163] The esterification can also be conducted with dioxygen as the terminal oxidant in the presence of a cooperative Ru-catalyst.[167] The group has also developed robust protocols for oxidative amidations and azidations,[103] Michael additions,[114] asymmetric synthesis of lactones[197] and indanes,[131] and enantioselective cyclopropanation of enals,[145] involving cascades comprising multiple C-C bond forming reactions.[227]
Development of Transition Metal Catalyzed Oxidative Coupling Reactions:
Modular Functionalization and Natural Product SynthesisInvestigations on transition metal catalyzed oxidative coupling reactions were initiated by the observation, that the TEMPO radical functions as an efficient oxidant in rhodium catalyzed direct C-H arylation using arylboronic acids.[76],[83] It was shown that these oxidative couplings can also be catalyzed by palladium,[88] allowing for arylations (e.g. of thiophenes;[118],[144] mechanistic studies:[139]). Oxidative arylations were subsequently expanded towards dearomatizing bisfunctionalization of heteroarenes (arylaminoxylation,[94],[109] acyloxyarylation[183],[211]). By using a similar approach oxidative Heck arylations[182] have been established. The potential of this route was documented e.g. by the stereoselective synthesis of Tamoxifen.[140] Stereospecific decarboxylative arylations in cyclohexadiene carboxylates have been developed,[130] evolving into a method for the regioselective preparation of highly substituted arenes.[165] These novel transformations have been successfully applied to the synthesis of resveratrol based natural products.[189]
The Electron as a Catalyst
Is the electron a catalyst in synthesis? This fundamental question is currently addressed in the Studer group. Bronsted acid catalysis is well established in organic synthesis. The electron, as compared to the proton about 1800 times smaller and omnipresent, is currently not recognized as a potential catalyst in synthesis. The concept of using the electron as a catalyst[201] is nearly unexplored, therefore, the group investigates the generality and broad applicability of the new concept, where reactions, which are generally conducted by using transition metals, are performed via electron catalysis.[203],[206]
Systems Chemistry –
Organic Chemistry at Interfaces and in Hybrid Materials
The basic knowledge gathered by the Studer group on synthetic radical chemistry is also successfully used in neighboring disciplines. For example the laboratory has significantly contributed to the field of Nitroxide Mediated controlled radical Polymerization (NMP). These activities have opened the door to a broader view on materials and to the application of organic transformations in meso scale systems. Moreover, with these lessons learned, the group is currently developing novel methods to chemically construct and modify molecular assemblies at the nano level in solution as well as at interfaces.
Read more: Systems Chemistry –
Organic Chemistry at Interfaces and in Hybrid MaterialsPerforming Chemical Reactions at Surfaces
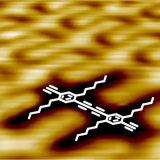
In a highly interdisciplinary approach the Studer group investigates chemical reactions at metal surfaces for the construction of extended covalently bond networks. These reactions often follow different pathways compared to analogous processes in solution. Reaction modes have been uncovered that have not been observed in solution. As a prototypical reaction, a Glaser-type coupling of conjugated alkynes has been achieved after spatial confinement on Ag(111)[161] and studies concerning the mechanism, photochemical initiation and selectivity have been performed.[173],[191],[313] On Cu(111) carbenes have been generated, characterized and also carbene coupling was established.[303] At the Au(111) surface a complex cascade comprising ortho C-H activation, C-C coupling and twofold cycloisomerization of aromatic nitriles to afford diazapyrenes has been developed.[337]
From NMP to Functional Materials
Over the past 15 years the group has continuously contributed to the development and application of nitroxide radicals for radical polymerization, in particular for nitroxide mediated polymerization (NMP). NMP is controlled by the persistent radical effect (PRE). Bulky nitroxides[42],[58],[64],[148],[194] serve as highly efficient initiators/regulators for controlled polymerization of alkenes like styrene,[61],[92] n-butyl acrylate or N-isopropyl acryl amide[146],[141] and for copolymerization reactions.s[123]. Methods for monitoring the polymerization process[187] have been developed as well.
The Studer group has consequently extended its work in polymer research towards the post modification of the polymer backbone: Transesterification protocols[184] using NHC catalysis[170] or photochemistry[157],[169] allow for the distinct installation of functional groups. Further protocols comprise e.g. the aminoxylation of allylic polymers,[113] aminoxylation and biofunctionalization of dendrimers[122] and the development of smart initiators.[115]
Significant contributions have been made to the field of chemical modification of nano and meso scale systems, which are constituted out of subunits of different scales with respect to size, polarity, and architecture.[185] As an example, the selective assembly[108] and (bio)chemical modification[120] of dye-loaded Zeolite containers has been achieved by chemical methods. In particular the nitroxide exchange reaction has been developed by the Studer group.[166] Initially, these reactions were utilized for the on-surface modification of polymers,[66] site selective polymerization at surfaces[70] for the generation of polymer brushes and polymer patterning at the nano scale.[91],[112],[143],[200] The nitroxide exchange reaction also allows for reversible chemical modification of self-assembled monolayers (SAMs).[129]
On the other hand, nitroxides and other radicals are incorporated into inorganic guest compounds, e.g. clay,[168] or aligned within polymeric grids.[164] These systems have been utilized as immobilized heterogeneous oxidation catalysts[101] or have been precisely aligned in order to study electronic effects arising from spin-spin coupling. Encapsulation of organic compounds has also been exploited for the generation of immobilized transition metal[68],[71],[177],[178] as well as organocatalyst systems.[82],[99] Immobilization of orthogonal addressable functionalized polymer residues on silica particles has been utilized for the generation of cooperatively acting catalyst systems for cross-coupling reactions[171] or Bronsted acid/base catalysis.[156]Applications towards Functional Bioconjugates
Polymers at surfaces eventually allow for controlled adjustment of height, patterning[192] or even site selection. Furthermore, the stimulus dependent aggregation behavior[119] can be utilized e.g. for triggering physiological processes, e.g. switching of enzyme activity.[74] Protein immobilization into structured polymer brushes was achieved by AFM lithography.[133] A combination of Langmuir–Blodgett pre structuring and orthogonal surface chemistry allowed patterning of proteins into nanostripes on Si-wafers,[116] and the effect of brush thickness of polystyrene brushes on protein adsorption was investigated by AFM-based force spectroscopy.[158] Vesicles as liposome analogues serve as supramolecular templates for the fabrication of polymer nanocontainers via cross-linking and subsequent core dissolution.[210]
