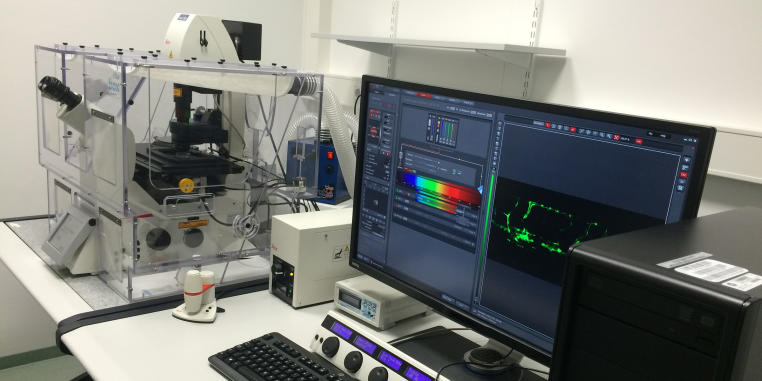

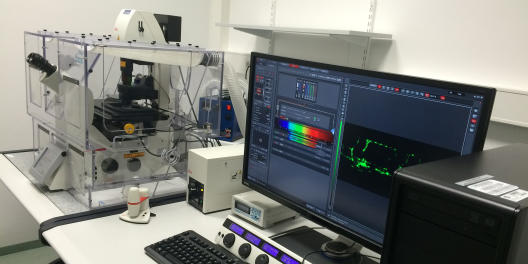
Leica TCS SP8
Location: Institute for Cardiovascular Organogenesis and Regeneration, Mendelstrasse 7
Group: AG Schulte-Merker
Contact Person: Dr. Laura Lleras Forero
Tel.: 0251 9802874, Laura.LlerasForero@ukmuenster.deApplications & Info:
- Life imaging with resonant scanner and environmental control, FRET and FRAP, advance tile scan, 3D projections.

© Uni MS - Institute of Neurobiology Leica TCS SP8-DLS laser scanning confocal microscope with “digital light sheet” module
Location: Institute of Neurobiology, Badestrasse 9
Group: AG Luschnig
Contact Person: Julia Sauerwald
Tel.: 0251 83-21695, sauerwaj@uni-muenster.deApplications & Info:
- Inverted microscope for high-resolution confocal imaging equipped with a resonant scanner and two HyD detectors for fast and sensitive acquisition.
- DLS module and Hamamatsu Orca Flash camera for light sheet imaging
- HyVolution software module for deconvolution postprocessing
- 405, 488, 552, and 638 nm laser lines
- 10x/0.40, 40x/1.30 and 63x/1.40 objectives

Olympus IX70 with TIRF objective and TILL Vision Monochromator
Location: Center for Molecular Biology of Inflammation (ZMBE), Von-Esmarch-Str. 56
Contact Person: Pia Brinkert
Tel.: 0251 83-53030, pia.brinkert@uni-muenster.deApplications & Info:
- Total internal reflection microscopy (TIRFM)
- TIRF and epifluorescence live-cell imaging (heating/CO2 incubation system connected)
- Only partially motorized!
- Runs on Metamorph Software
- Excitation lines: 488 nm, 568 nm
- Objectives: 10x/0.30, 20x/0.75, 40x/0.75, 60x/TIRFM/1.45/Oil, 100x/1.35/Oil

© Institute of Physiology I Olympus FluoView 1000 / laser scanning confocal microscope
Location: Institute of Physiology I
Group: AG Pape
Contact Person: Dr. Peter Blaesse
Tel.: 0251 83-55416, blaesse@uni-muenster.de
Applications & Info:- optimized for imaging of acute tissue slices
- combined imaging/electrophysiology (patch clamp) setup
- suitable for slice optogenetics and uncaging experiments (488 nm, 514 nm, 543 nm)

© ZMBE Zeiss Axio Imager Z1 with spinning disc confocal microscope
Location: Institute for cell biology
Group: AG Raz
Contact Person: Łukasz Truszkowski
l_trus01@wwu.de, Tel. lab: 0251 83-58618, Tel. office: 0251 83-52110
Applications & Info:- Fluorescence imaging, Confocal imaging
- Upright microscope capable of confocal imaging, equipped with water-immersive objectives. The system is equipped with Spinning disc and Hamamatsu camera, there are additional 405nm and 355nm laser for photo bleaching and laser ablation experiments.

Zeiss Axio Observer.Z1 spinning disc confocal microscope
Location: Center for Molecular Biology of Inflammation (ZMBE), Von-Esmarch-Str. 56
Contact Person: Matteo Rizzato
Tel.: 0251 83-53030, rizzato@uni-muenster.deApplications & Info:
- Long-term live cell imaging possible (VisiScope Cell Explorer Life Cell Imaging System, Zeiss Z1 Axio Observer Z1 stage, Yokogawa CSU22 spinning disc head, incl. incubation chamber, heating, CO2; Zeiss definite focus)
- Motorized stage with autofocus (Zeiss definite focus)
- Runs on Metamorph Software
- Microscopy in optical bottom 96-well plates possible
- Excitation lines: 488 nm, 561 nm, 640 nm
- Objectives: 20x, 40x oil, 60x oil

© ZMBE Zeiss LSM 710 confocal microscope
Location: Institute for cell biology
Group: AG Raz
Contact Person: Łukasz Truszkowski
l_trus01@wwu.de, Tel. lab: 0251 83-58618, Tel. office: 0251 83-52110
Applications & Info:- Fluorescence imaging
- Upright microscope capable of confocal imaging, Equipped with water merged objectives. The system is equipped with 2photon system (Coherent,chameleon, 690nm to 1100nm lasers).
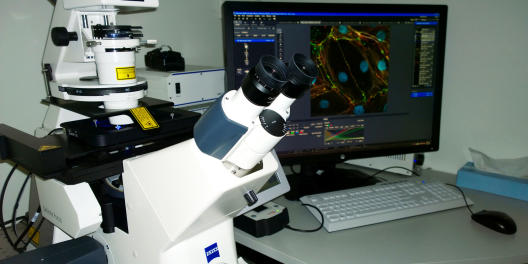
Zeiss LSM 800 with Airyscan
Location: Institute of Medical Biochemistry (ZMBE), Von-Esmarch-Str. 56
Contact Persons: Sophia Koerdt, Tel.: 0251 83-52123, s.koerdt@uni-muenster.de
Christian Hartmann, Tel.: 0251 83-52120, c_hart11@uni-muenster.deApplications & Info:
- Confocal laser scanning microscope with high resolution and enhanced sensitivity
- Inverted stand: Axio Observer.Z1
- Motorized stage with piezo insert
- Excitation lines: 405, 488, 561 and 640 nm
- Objective lense: 63X/1.4 Oil DIC
- 2 GaAsP detectors, 1 Airyscan GaAsP detector, transmitted light detector
- In Airyscan mode 1,7X better resolution in all three dimensions compared to standard confocal imaging

© Institute of Anatomy and Molecular Neurobiology VisiFluor High Performance Imaging and Electrophysiology set-up - Zeiss Axio Observer.A1
Location: Institute of Anatomy and Molecular Neurobiology, Vesaliusweg 2-4 (PAN-Zentrum)
Contact Person: Dr. Johannes Brockhaus
Tel.: 0251 83-50203, j.brockhaus@uni-muenster.de
Applications & Info:- imaging set-up, optimized for fast Ca2+-measurements
- digital sCMOS camera „Hamamatsu ORCA-Flash4.0 V2“ (2 Mpixel)
- microscope LED-illumination system „lumencor SPECTRA X light engine“
- high speed polychromatic illumination system „VisiChrome“
- 2D-VisiFRAP Galvo system inc. VS laser module (405nm) for fluorescence recovery / photobleaching
- different Chroma ET Filter-Sets
- objectives: 10x/0,25; 40x/1,2; 63x/1,2
- patch clamp amplifier „HEKA EPC 10 double“
- high current isolator „WPI A385“ for field stimulation

© Institute of Anatomy and Molecular Neurobiology VisiScope cell analyser / fluorescence microscope - Zeiss Axio Imager.Z2
Location: Institute of Anatomy and Molecular Neurobiology, Vesaliusweg 2-4 (PAN-Zentrum)
Contact Person: Dr. Christian Neupert
Tel.: 0251 83-50235, cneupert@uni-muenster.de
Applications & Info:- deep-frozen fluorescence camera „Diagnostic Instruments Spot-Xplorer“ (1,4 Mpixel)
- high-resolution color camera „Diagnostic Instruments Spot-Flex“ (up to 16 Mpixel)
- objectives: 5x/0,16; 10x/0,3; 20x/0,8; 40x/0,75; 40x/1,30 oil; 63x/1,40 oil; 100x/1,46 oil
- joystick and software controlled, motorized microscope stage for 8 slides
- MetaMorph imaging software (6D-multidimensional presentation)

© Institute of Anatomy and Molecular Neurobiology VisiScope spinning disc confocal microscope for live cell imaging - Zeiss Axio Observer.Z1
Location: Institute of Anatomy and Molecular Neurobiology, Vesaliusweg 2-4 (PAN-Zentrum)
Contact Person: Dr. Christian Neupert
Tel.: 0251 83-50235, cneupert@uni-muenster.de
Applications & Info:- long-term live cell imaging (VisiScope Cell Explorer Life Cell Imaging System)
- spinning disc confocal scanning head „Yokogawa CSU X1“
- VS laser module with 4 exitation lines (405nm, 488nm, 561nm, 640nm)
- incubation chamber for local CO2 gassing
- objectives: 10x/0,3; 20x/0,8; 40x/1,40 oil; 63x/1,40 oil

