Chemists present method for the fluorination of enynes
Fluorinated small molecules are very often used to produce medicines or agrochemicals. However, they rarely occur naturally. The societal importance of fluorinated substances, combined with the lack of natural sources, has created a demand for effective, sustainable methods to generate new fluorinated motifs – molecular structures containing one or more fluorine atom – from relatively simple starting materials. A team led by chemist Prof. Ryan Gilmour from the University of Münster now shows how a metal-free, organocatalytis platform can be used to fluorinate so-called enynes, a group of special carbon compounds. The study is published in the scientific journal "Nature Chemistry".
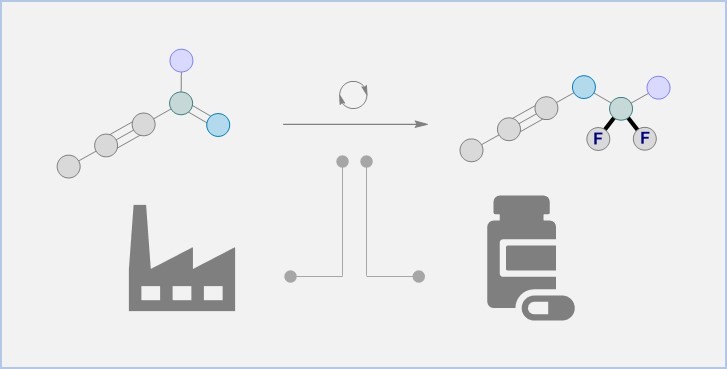
The new synthesis method produces a wide range of difluorinated molecular building blocks that can be candidates for new active substances. "We use simple enynes, which are largely composed of carbon and hydrogen," Ryan Gilmour describes. "Our strategy gets around an important limitation in difluorination chemistry. It allows some of the substrate to migrate or rearrange itself during the process. This is a bit like molecular origami, where we can reproducibly expose a simple starting material to our catalysis conditions and create a more complex product with two new carbon-fluorine bonds." The process is operationally simple and compatible with small molecule drugs: the team shows one example of the modified drug Febuxostat, which is used to treat hyperuricaemia - excessive levels of uric acid in the blood - and gout. Dr. Zi-Xuan Wang, lead author of the study, also achieved the shortest synthesis of difluoropalmitic acid known to date, which underscores the efficiency and utility of the approach.
Background: The importance of fluorinated substances in medicine can be illustrated by work from the late 1950s. At that time, the improved performance of fluorinated steroids compared to their non-fluorinated counterparts was demonstrated: The anti-inflammatory glucocorticoid dexamethasone is one example. It is similar in structure to cortisol, which is found in the body, but unlike it contains fluorination - and is around 25 times more potent.
Original publication
Wang, ZX., Livingstone, K., Hümpel, C. et al. Regioselective, catalytic 1,1-difluorination of enynes. Nat. Chem. (2023); DOI: 10.1038/s41557-023-01344-5
