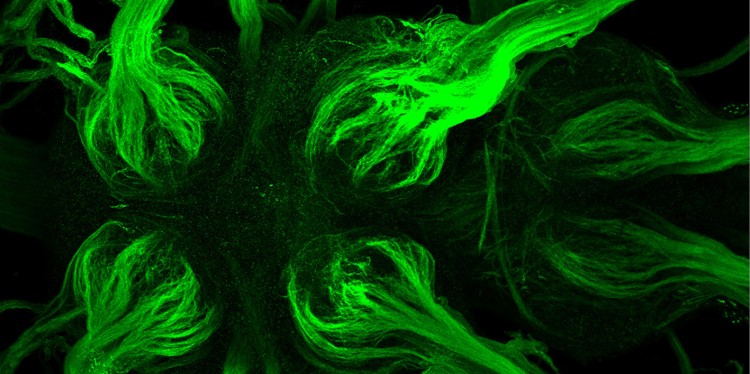
Researchers find structures enabling a rapid transmission of nerve impulses in insects
An animal’s brain consists of two different types of cell: neurons, which process and transmit information, and glial cells, which support the neurons in a variety of ways. In 1871, the French anatomist Louis-Antoine Ranvier demonstrated something special about neurons in vertebrates: on the extensions of these nerve cells there are ring-shaped regions which lack a surrounding sheath – the myelin formed by glial cells. Together with the electrically insulating myelin sheath, the so-called nodes of Ranvier form a basis for electrical nerve impulses to be transferred very rapidly over longer distances. They “jump” from node to node at a speed of up to 100 metres per second. This “saltatory conduction” was long seen as being specific to vertebrates. Now, for the first time, taking the fruit fly (Drosophila melanogaster) as an example, a team of researchers led by neurobiologist Prof. Christian Klämbt from the Institute for Neuro- and Behavioural Biology at Münster University has shown that similar structures exist in insects. The study has been published in the journal eLife.


The work done by the team not only helps in understanding the evolution of myelin, but also enables the biology of myelin formation and regeneration to be investigated in greater detail. This is of major importance in connection with neurodegenerative diseases such as multiple sclerosis. So far, treatment has focused on suppressing the inflammatory reaction, and it has not been possible to promote any effective re-myelinisation. “This means,” says Christian Klämbt, “that our findings will help in discovering alternative forms of treatment for multiple sclerosis.”
For their investigations, the researchers used methods of molecular genetics in combination with various state-of-the-art imaging processes, including the use of innovative electron microscopy for the visualisation of marked proteins, as well as confocal microscopy with especially high resolutions.
The German Research Foundation (DFG) provided financial support (SFB 1348, B5, Kl 588/29).
Original publication
Simone Rey, Henrike Ohm, Frederieke Moschref, Dagmar Zeuschner, Marit Praetz, and Christian Klämbt (2023): Glial-dependent clustering of voltage-gated ion channels in Drosophila precedes myelin formation. DOI: 10.7554/eLife.85752
