
Study shows new way to produce important molecular entity
Among the most common structures relevant to the function of biologically active molecules, natural products and drugs are so-called vicinal diamines - in particular, unsymmetrically constructed diamines. Vicinal diamines contain two functional atomic groups responsible for the substance properties, each with a nitrogen atom bonded to two neighbouring carbon atoms. A team led by Prof. Dr. Frank Glorius of the Institute of Organic Chemistry at the University of Münster has now presented a new, direct way to produce vicinal diamines in the journal "Nature Catalysis".
In contrast to other, less suitable methods, the process does not require the use of transition metals and iodine reagents as catalysts. Instead, the researchers use light energy to produce the desired diamines from various electron-rich aromatic hydrocarbons (arenes and heteroarenes). "In this way, we obtain a series of vicinal diamines that were previously difficult to produce. In doing so, we can precisely control the sites where the functional groups are located," explains first author Dr. Guangying Tan.
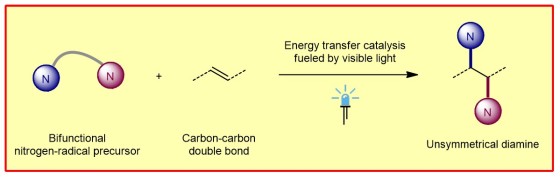
"The molecules of life consist largely of carbon chains and rings of varying size and complexity. The decoration of these 'plain' chains with other elements is crucial for the resulting properties of these compounds," Frank Glorius explains the background. A key role is played by the elements oxygen and nitrogen. Chemists refer to these non-carbon elements as heteroatoms. "Methods for the efficient and controlled introduction of these heteroatoms into artificially produced, biologically active structures are therefore of great importance," Frank Glorius emphasizes. "This also applies to the vicinal diamines we are focusing on."
The chemists perform the diamination reaction under irradiation with blue light-emitting diodes (LEDs) and using an inexpensive and commercially available thioxanthone as an organic photosensitizer.
The study was financially supported by the Alexander von Humboldt Foundation, the Chemical Industry Fund (106151) and the German Research Foundation (SFB 858).
Original publication
Guangying Tan, Mowpriya Das, Roman Kleinmans, Felix Katzenburg, Constantin Daniliuc, and Frank Glorius (2022): Energy Transfer-Enabled Unsymmetrical Diamination Using Bifunctional Nitrogen-Radical Precursors. Nature Catalysis; DOI: 10.1038/s41929-022-00883-3
