Green tea-based food supplements

The evergreen tea shrub (Camellia sinsensis (L.) Kuntze, Fig. 1) is a plant from the Theaceae family and is known to be rich in polyphenols. Tea leaves can contain up to 30% of the dry weight of phenolic ingredients [1].
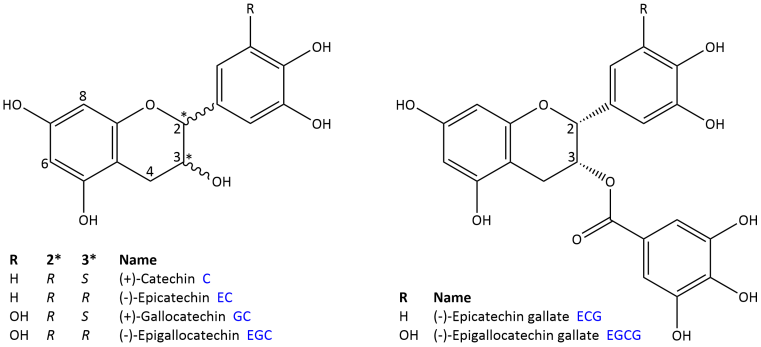
Fresh tea leaves and green tea contain large amounts of monomeric flavanols (Fig. 2) and their 3-O-gallates. These compounds are sometimes abbreviated to "tea catechins". Green tea extracts are dominated by the compound (-)-epigallocatechin gallate (up to 12% [1]), which has become well known under the name "EGCG" (Fig. 2). Other compounds that occur in significant amounts are: (-)-epicatechin gallate (ECG), (-)-epicatechin (EC) and (-)-epigallocatechin (EGC). Dimeric and trimeric proanthocyanidins are also present, which are made up of linked catechin, epicatechin and epigallocatechin units, as well as gallic acid esters of these compounds. During fermentation, tannin-like oxidation products (theaflavins, theaflagallins and thearubigens) are formed from the galloylated compounds in particular, which are the characteristic colouring agents of black tea [1].
A chemopreventive effect has been demonstrated for various types of cancer in a large number of in vitro and in vivo experiments as well as in epidemiological studies. The anticarcinogenic effect in green tea seems to be primarily due to EGCG, which is largely degraded during fermentation [1].
Case report: Concentrated green tea extract induces severe acute hepatitis in a 63-year-old woman.
This case report describes treatment with green tea capsules on the recommendation of a cancer support group. It joins the small but growing number of reports of liver toxicity associated with supplements containing high doses of EGCG and highlights the fact that such concentrated green tea extracts are not free of adverse effects.
We were able to verify the manufacturer's claims with a U(H)PLC UV method (see case report). The analysis of the capsule contents showed no evidence of other foreign ingredients and proved the authenticity of the food supplement "Mega Green Tea Extract". One capsule contained 725 mg of decaffeinated green tea extract with a 45% content of EGCG. See chromatogram.
Following several cases of liver damage possibly caused by highly concentrated green tea extracts, the European Food Safety Authority (EFSA) has assessed the safety of green tea and green tea extracts [2]. The panel concludes that "catechins" from traditionally prepared green tea infusion are generally considered safe. At the same time, it highlights that there is evidence to show that taking EGCG at a dose of 800 mg/day or more as a food supplement causes a statistically significant increase in serum transaminases in treated subjects compared to the control group. Thus, there seems to be no evidence that consumption of green tea as a beverage can lead to hepatotoxicity.
Based on the literature and our observation, green tea extracts in the form of food supplements require attention because of possible side effects. In addition to appropriate labelling of these products, pharmaceutical advice and medical monitoring should be indicated. Any increase in liver enzymes should call into question the use of green tea extracts. The products should then be discontinued immediately.
Literature
[1] R. Hänsel - O. Sticher (eds.) Pharmacognosy - Phytopharmacy, 9th revised and updated edition, Springer Medizin Verlag Heidelberg, ISBN 978-3-642-00962-4.
[2] EFSA Journal (2018) Scientific opinion on the safety of green tea catechins.


