The SupraPhoto group Publications (chronological order).
26.
Electron Donor–Acceptor Complexes Without Preinstalled Sacrificial Leaving Groups: Applications in Synthesis,
W. Liu, L. Næsborg,
Helv. Chim. Acta 2026, 108, e00168. [doi:10.1002/hlca.202500168]
W. Liu, L. Næsborg,
Helv. Chim. Acta 2026, 108, e00168. [doi:10.1002/hlca.202500168]
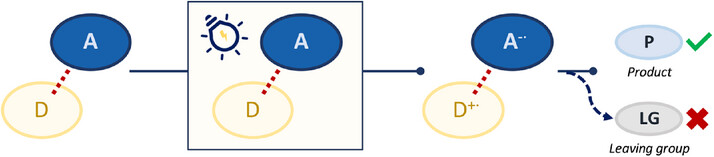
25.
Long-lived room temperature phosphorescence in aqueous micellar systems: application in cyclobutane synthesis,
G. Pölderl, J. C. G. Kürschner, A. Kondrateva, A. Albers, M. Schönhoff, F. Rizzo, L. Næsborg ,
J. Mater. Chem. A 2025, 13, accepted. [doi:10.1039/D5TA05567A]
G. Pölderl, J. C. G. Kürschner, A. Kondrateva, A. Albers, M. Schönhoff, F. Rizzo, L. Næsborg ,
J. Mater. Chem. A 2025, 13, accepted. [doi:10.1039/D5TA05567A]
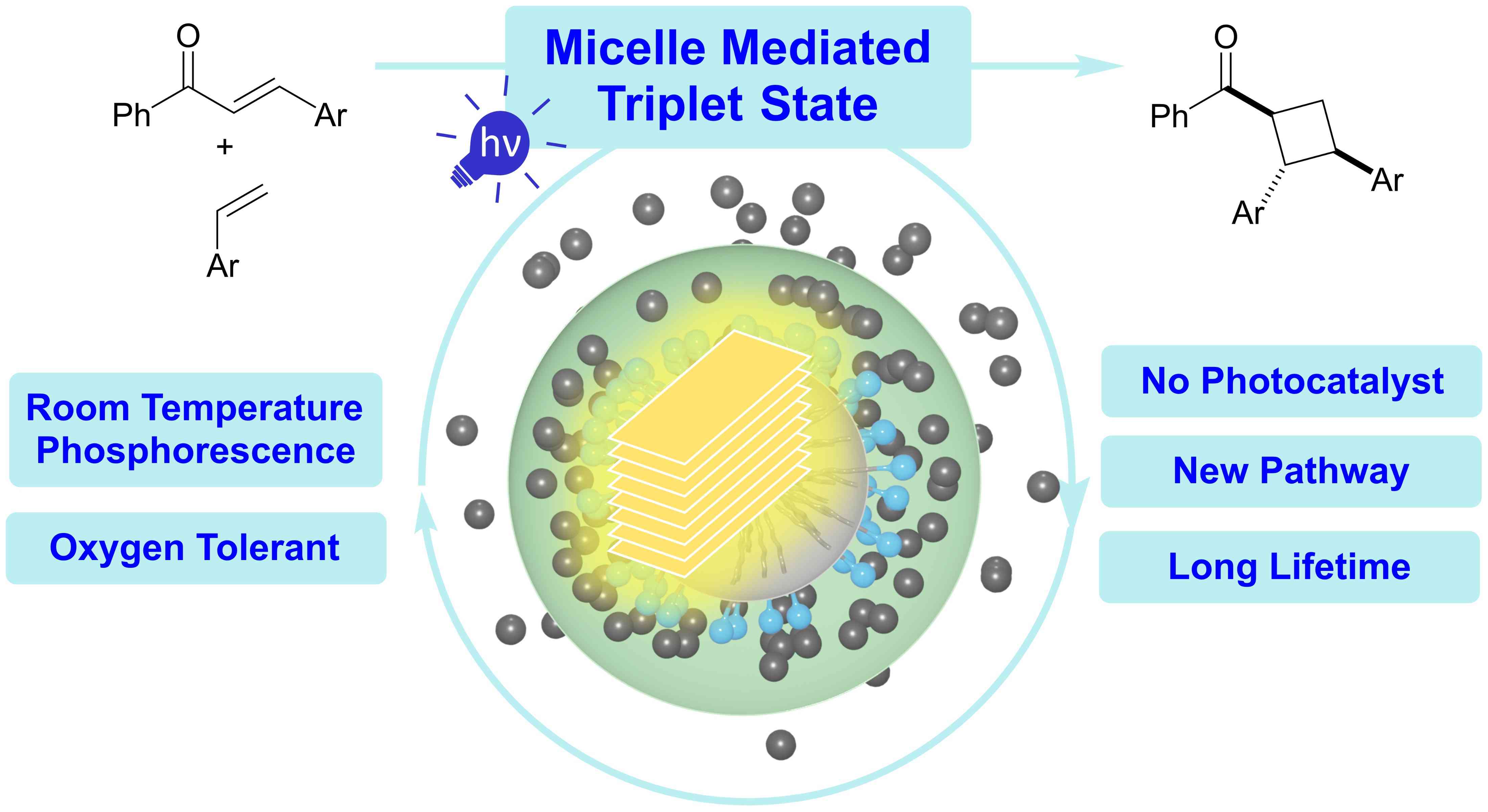
24.
Regiodivergent Photocatalytic Annulation for the Synthesis of gem-Difluorinated Cyclic Hydrocarbons,
T. E. Schirmer, J. C. G. Kürschner, Y. Uchida, Y. Taura, P. Gabriel, L. Næsborg, D. Yokogawa, Y. Aramaki, T. Ooi,
Angew. Chem. Int. Ed. 2025, 64, e202502450. [doi:10.1002/anie.202502450]
T. E. Schirmer, J. C. G. Kürschner, Y. Uchida, Y. Taura, P. Gabriel, L. Næsborg, D. Yokogawa, Y. Aramaki, T. Ooi,
Angew. Chem. Int. Ed. 2025, 64, e202502450. [doi:10.1002/anie.202502450]
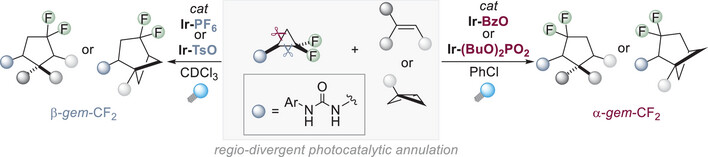
23.
EDA complexation of two donor molecules initiating radical cation cyclizations,
J. Kürschner, M. Utikal, L. Lezius, R. Jeyaseelan, L. D. Næsborg,
Synthesis 2025, 57, 2105-2114. [doi:10.1055/a-2518-0987]
J. Kürschner, M. Utikal, L. Lezius, R. Jeyaseelan, L. D. Næsborg,
Synthesis 2025, 57, 2105-2114. [doi:10.1055/a-2518-0987]
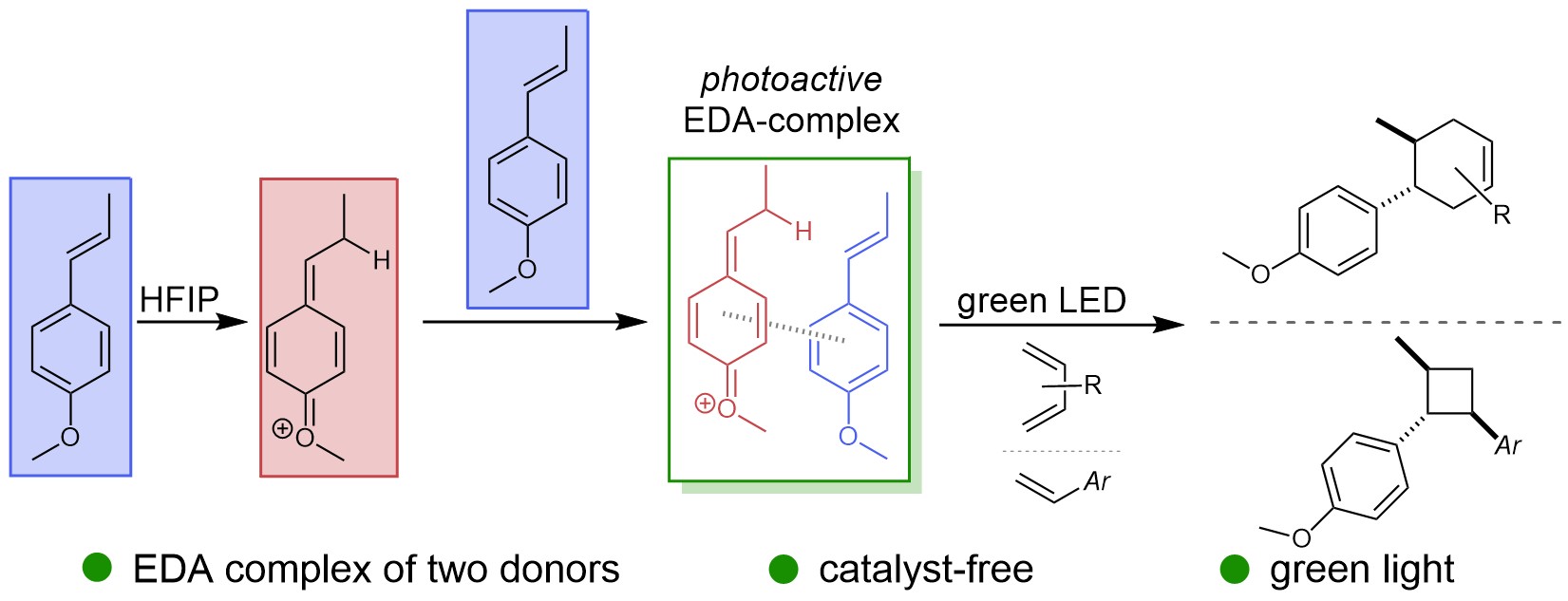
22.
Methyl viologen as a catalytic acceptor for electron donor-acceptor photoinduced cyclization reactions ,
R. Jeyaseelan, W. Liu, J. Zumbusch, L. D. Næsborg,
Green Chem. 2024, 26, 1969-1973. [doi:10.1039/D4GC05481D]
R. Jeyaseelan, W. Liu, J. Zumbusch, L. D. Næsborg,
Green Chem. 2024, 26, 1969-1973. [doi:10.1039/D4GC05481D]
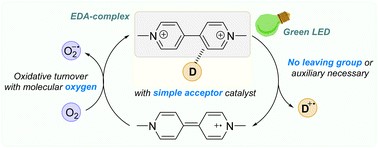
21.
Triplet-triplet annihilation upconversion,
L. Næsborg, R. Jeyaseelan,
Nachr. Chem. 2024, 72, 62-64. [doi:10.1002/nadc.20244142065]
L. Næsborg, R. Jeyaseelan,
Nachr. Chem. 2024, 72, 62-64. [doi:10.1002/nadc.20244142065]
20.
Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop,
S. B.Beil, S. Bonnet, C. Casadevall, R. J. Detz, F. Eisenreich, S. D. Glover, C. Kerzig, L. Næsborg, S. Pullen, G. Storch, N. Wie, C. Zeymer,
JACS Au 2024, 4, 2746-2766. [doi:10.1021/jacsau.4c00527]
S. B.Beil, S. Bonnet, C. Casadevall, R. J. Detz, F. Eisenreich, S. D. Glover, C. Kerzig, L. Næsborg, S. Pullen, G. Storch, N. Wie, C. Zeymer,
JACS Au 2024, 4, 2746-2766. [doi:10.1021/jacsau.4c00527]
19.
Blickpunkt Synthese: Photochemical Synthesis in Water,
L. Næsborg,
Nachr. Chem. 2024, 72, 68-70. [doi:10.1002/nadc.20244142061]
L. Næsborg,
Nachr. Chem. 2024, 72, 68-70. [doi:10.1002/nadc.20244142061]
18.
Avoiding Oxygen Removal for Photochemical Reactions – towards Water as the Solvent,
G. Pölderl, L. Næsborg,
ChemPhotoChem 2024, 8, e202300340. [doi:10.1002/cptc.202300340]
G. Pölderl, L. Næsborg,
ChemPhotoChem 2024, 8, e202300340. [doi:10.1002/cptc.202300340]
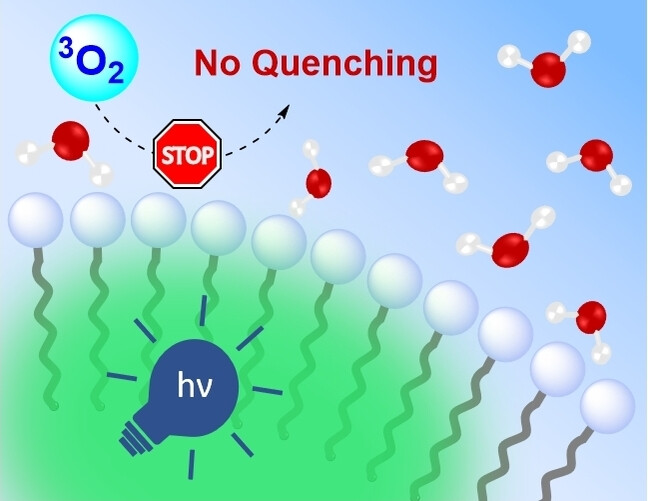
17.
UV-Light Generation in Micelles via Triplet-Triplet Annihilation Upconversion – Towards Synthetic Applications,
R. Jeyaseelan, M. Utikal, L. Næsborg,
Asian J. Org. Chem 2024, 13, e202300604. [doi:10.1002/ajoc.202300604]
R. Jeyaseelan, M. Utikal, L. Næsborg,
Asian J. Org. Chem 2024, 13, e202300604. [doi:10.1002/ajoc.202300604]
16.
Photocyclization by a triplet–triplet annihilation upconversion pair in water – avoiding UV-light and oxygen removal,
R. Jeyaseelan, M. Utikal, C. G. Daniliuc, L. Næsborg,
Chem. Sci. 2023, 14, 11040-11044. [doi:10.1039/D3SC03242F]
R. Jeyaseelan, M. Utikal, C. G. Daniliuc, L. Næsborg,
Chem. Sci. 2023, 14, 11040-11044. [doi:10.1039/D3SC03242F]
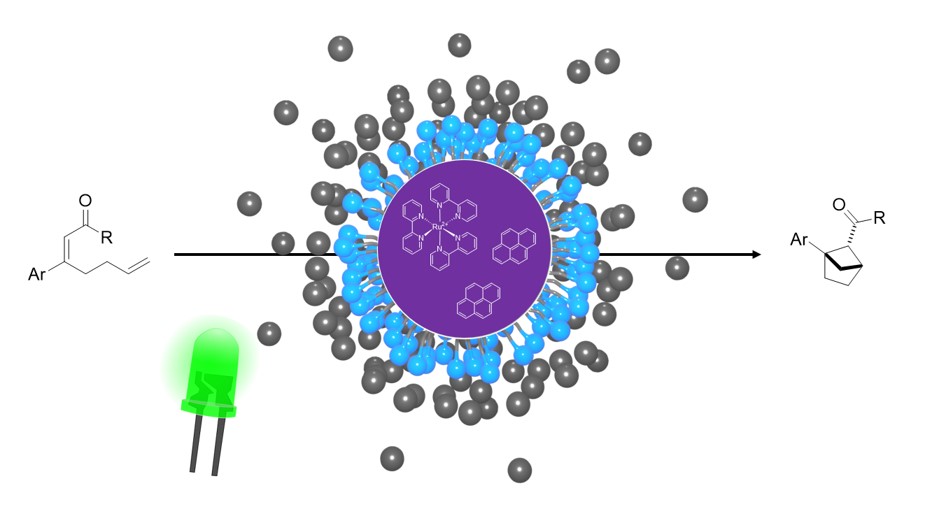
15.
Special Collection: Photocatalytic Synthesis,
L. Næsborg, B. Pieber, O. S. Wenger,
ChemCatChem 2023, 15, e202300683. [doi:10.1002/cctc.202300683]
L. Næsborg, B. Pieber, O. S. Wenger,
ChemCatChem 2023, 15, e202300683. [doi:10.1002/cctc.202300683]
14.
Facilitating [2+2] Photocycloadditions by Promoting Oxygen Tolerance and Substrate Activation in Water,
J. C. G. Kürschner, L. Brüss, L. Næsborg,
Chem. Eur. J. 2023, 29, e202300627. [doi:10.1002/chem.202300627];
highlighted in Chem Catalysis 2023, 3, 100861.
J. C. G. Kürschner, L. Brüss, L. Næsborg,
Chem. Eur. J. 2023, 29, e202300627. [doi:10.1002/chem.202300627];
highlighted in Chem Catalysis 2023, 3, 100861.
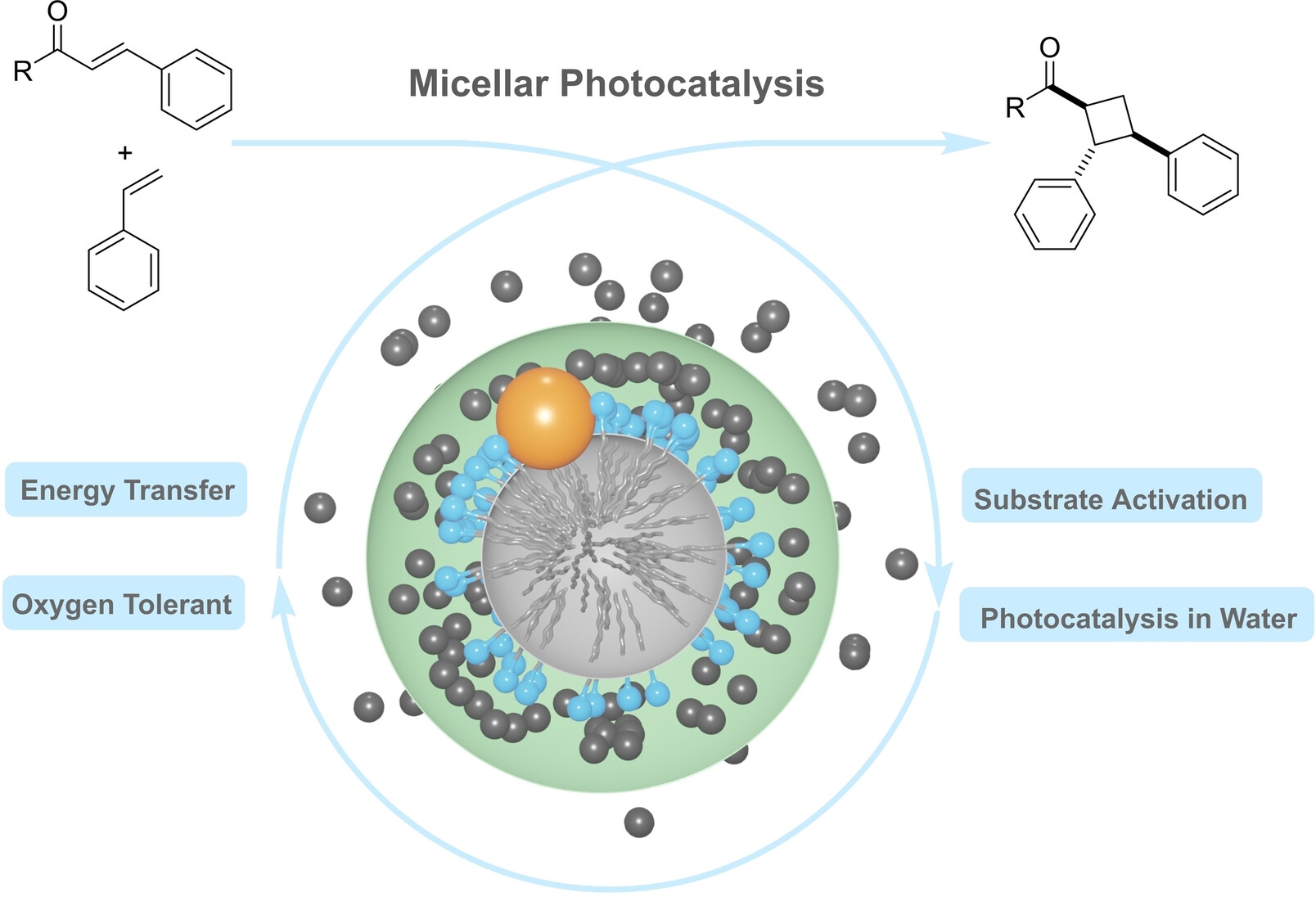
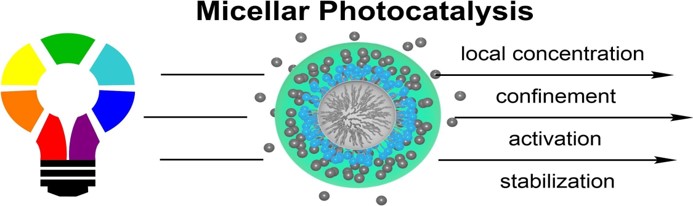
13.
Micellar Effects and their Relevance in Photochemistry and Photocatalysis,
L. Brüss, R. Jeyaseelan, J. C. G. Kürschner, M. Utikal,L. Næsborg,
ChemCatChem 2022, 15, e202201146. [doi:10.1002/cctc.202201146]
L. Brüss, R. Jeyaseelan, J. C. G. Kürschner, M. Utikal,L. Næsborg,
ChemCatChem 2022, 15, e202201146. [doi:10.1002/cctc.202201146]
12.
Concise Total Synthesis of Agarozizanol B via a Strained Photocascade Intermediate,
N. Rauscher, L. Næsborg, C. Jandl, T. Bach,
Angew. Chem. Int. Ed. 2021, 60, 24039-24042. [doi:10.1002/anie.202110009]
N. Rauscher, L. Næsborg, C. Jandl, T. Bach,
Angew. Chem. Int. Ed. 2021, 60, 24039-24042. [doi:10.1002/anie.202110009]
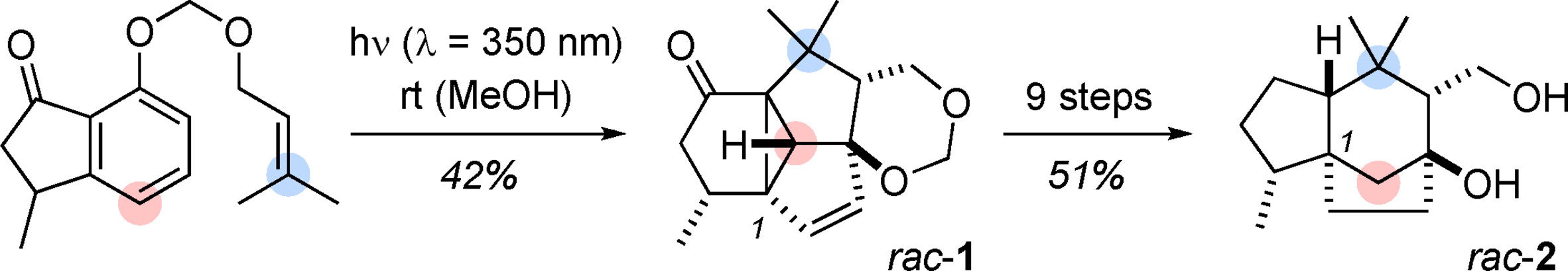
11.
Stereoselective Oxidative Bioconjugation of Amino Acids and Oligopeptides to Aldehydes,
H. N. Tobiesen, L. A. Leth, M. V. Iversen, L. Næsborg, S. Bertelsen, K. A. Jørgensen,
Angew. Chem. Int. Ed. 2020, 59, 18490-18494. [doi:10.1002/anie.202008513]
H. N. Tobiesen, L. A. Leth, M. V. Iversen, L. Næsborg, S. Bertelsen, K. A. Jørgensen,
Angew. Chem. Int. Ed. 2020, 59, 18490-18494. [doi:10.1002/anie.202008513]
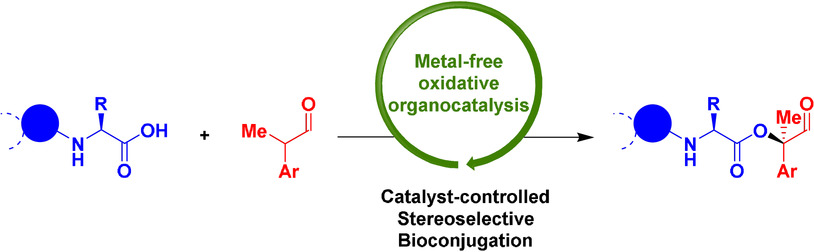
10.
Complex Carbocyclic Skeletons from Aryl Ketones through a Three-Photon Cascade Reaction,
L. Næsborg, C. Jandl, A. Zech, T. Bach,
Angew. Chem. Int. Ed. 2020, 59, 5656-5659. [doi:10.1002/anie.201915731]
L. Næsborg, C. Jandl, A. Zech, T. Bach,
Angew. Chem. Int. Ed. 2020, 59, 5656-5659. [doi:10.1002/anie.201915731]
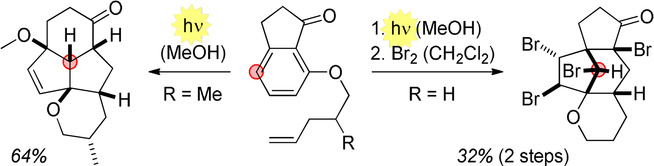
09.
Oxidative organocatalysed enantioselective coupling of indoles with aldehydes that forms quaternary carbon stereocentres,
N. M. Rezayee, V. H. Lauridsen, L. Næsborg, T. V. Q. Nguyen, H. N. Tobiesen, K. A. Jørgensen,
Chem. Sci 2019, 10, 3586-3591. [doi:10.1039/C9SC00196D]
N. M. Rezayee, V. H. Lauridsen, L. Næsborg, T. V. Q. Nguyen, H. N. Tobiesen, K. A. Jørgensen,
Chem. Sci 2019, 10, 3586-3591. [doi:10.1039/C9SC00196D]
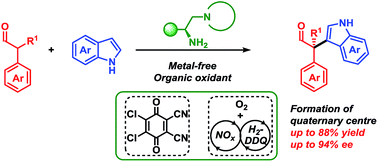
08.
Enantioselective Oxidative Coupling of Carboxylic Acids to α-Branched Aldehydes,
L. L. Leth, L. Næsborg, G. J. Reyes-Rodríguez, H. N. Tobiesen, M. V. Iversen, K. A. Jørgensen,
J. Am. Chem. Soc. 2018, 140, 12687-12690. [doi:10.1021/jacs.8b07394]
L. L. Leth, L. Næsborg, G. J. Reyes-Rodríguez, H. N. Tobiesen, M. V. Iversen, K. A. Jørgensen,
J. Am. Chem. Soc. 2018, 140, 12687-12690. [doi:10.1021/jacs.8b07394]
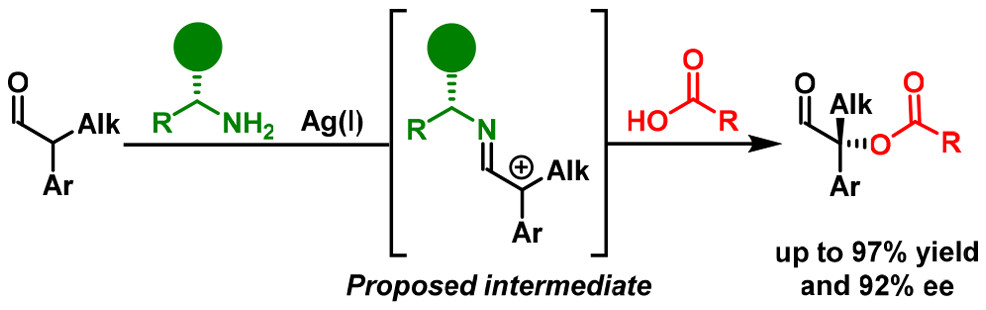
07.
Direct Enantio‐ and Diastereoselective Oxidative Homocoupling of Aldehydes,
L. Næsborg, L. A. Leth, G. J. Reyes-Rodríguez, T. A. Palazzo, V. Corti, K. A. Jørgensen,
Chem. Eur. J. 2018, 24, 14844-14848. [doi:10.1002/chem.201803506]
L. Næsborg, L. A. Leth, G. J. Reyes-Rodríguez, T. A. Palazzo, V. Corti, K. A. Jørgensen,
Chem. Eur. J. 2018, 24, 14844-14848. [doi:10.1002/chem.201803506]
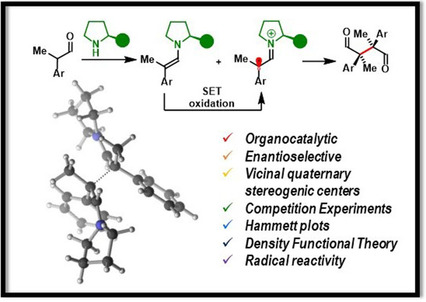
06.
Catalytic Asymmetric Oxidative γ‐Coupling of α,β‐Unsaturated Aldehydes with Air as the Terminal Oxidant,
L. Næsborg, V. Corti, L. A. Leth, P. H. Poulsen. K. A. Jørgensen,
Angew. Chem. Int. Ed. 2018, 57, 1606-1610. [doi:10.1002/anie.201711944]
L. Næsborg, V. Corti, L. A. Leth, P. H. Poulsen. K. A. Jørgensen,
Angew. Chem. Int. Ed. 2018, 57, 1606-1610. [doi:10.1002/anie.201711944]
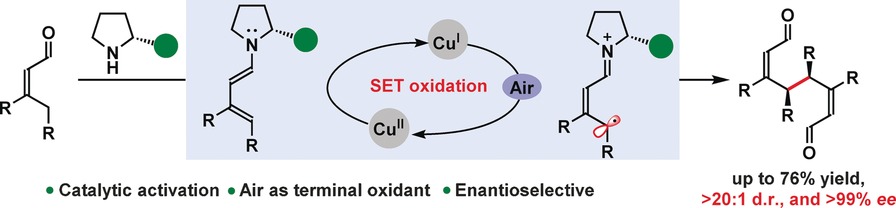
05.
Synergistic Catalysis for the Asymmetric [3+2] Cycloaddition of Vinyl Aziridines with α,β‐Unsaturated Aldehydes,
L. Næsborg, F. Tur, M. Meazza, J. Blom, K. S. Halskov, K. A. Jørgensen,
Chem. Eur. J. 2017, 23, 268-272. [doi:10.1002/chem.201604995]
L. Næsborg, F. Tur, M. Meazza, J. Blom, K. S. Halskov, K. A. Jørgensen,
Chem. Eur. J. 2017, 23, 268-272. [doi:10.1002/chem.201604995]
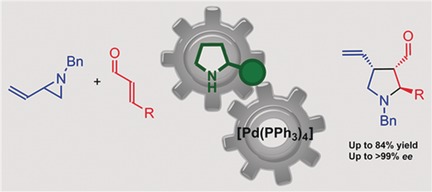
04.
Enantioselective Organocatalytic Cascade Approach to Different Classes of Benzofused Acetals,
B. M. Paz, L. Klier, L. Næsborg, V. H. Lauridsen, F. Jensen, K. A. Jørgensen,
Chem. Eur. J. 2016, 22, 16810-16818. [doi:10.1002/chem.201602992]
B. M. Paz, L. Klier, L. Næsborg, V. H. Lauridsen, F. Jensen, K. A. Jørgensen,
Chem. Eur. J. 2016, 22, 16810-16818. [doi:10.1002/chem.201602992]
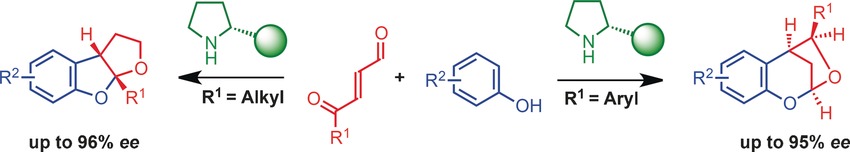
03.
Asymmetric [3 + 2] Cycloaddition of Vinylcyclopropanes and α,β-Unsaturated Aldehydes by Synergistic Palladium and Organocatalysis,
K. S. Halskov, L. Næsborg, F. Tur, K. A. Jørgensen,
Org. Lett. 2016, 18, 2220-2223. [doi:10.1021/acs.orglett.6b00852]
K. S. Halskov, L. Næsborg, F. Tur, K. A. Jørgensen,
Org. Lett. 2016, 18, 2220-2223. [doi:10.1021/acs.orglett.6b00852]
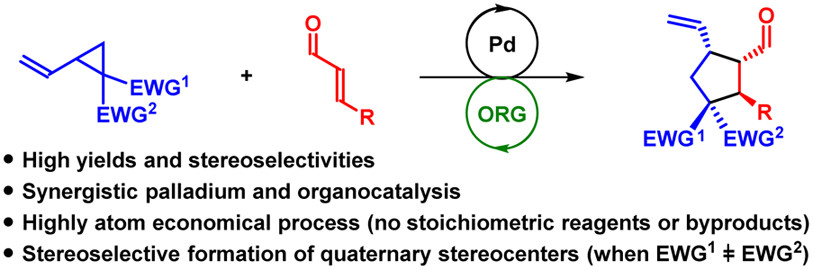
02.
Asymmetric γ‐Allylation of α,β‐Unsaturated Aldehydes by Combined Organocatalysis and Transition‐Metal Catalysis,
L. Næsborg, K. S. Halskov, F. Tur, S. M. N. Mønsted, K. A. Jørgensen,
Angew. Chem. Int. Ed. 2015, 54, 10193-10197. [doi:10.1002/anie.201504749]
L. Næsborg, K. S. Halskov, F. Tur, S. M. N. Mønsted, K. A. Jørgensen,
Angew. Chem. Int. Ed. 2015, 54, 10193-10197. [doi:10.1002/anie.201504749]
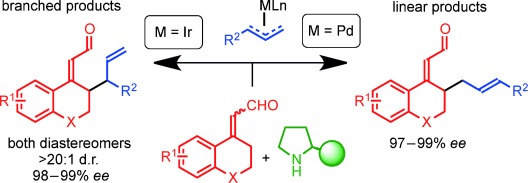
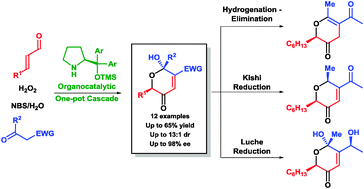
01.
An organocatalytic one-pot cascade incorporating the Achmatowicz reaction affording 3-pyrone derivatives,
L. K. Ransborg, L. Lykke, N. Hammer, L. Næsborg, K. A. Jørgensen,
Chem. Commun. 2014, 50, 7604-7606. [doi:10.1039/C4CC03815K]
L. K. Ransborg, L. Lykke, N. Hammer, L. Næsborg, K. A. Jørgensen,
Chem. Commun. 2014, 50, 7604-7606. [doi:10.1039/C4CC03815K]