

Organohalogen Chemistry
The Hennecke group is interested in research in the areas of organic and bioorganic chemistry. A key component of research focuses on organohalogen compounds, especially of the heavier halogens chlorine, bromine and iodine. Since such compounds occur as natural products and are valuable intermediates in organic synthesis, the group is developing catalytic asymmetric methods for the regio- and stereoselective synthesis of these compounds.
Selected Publications:
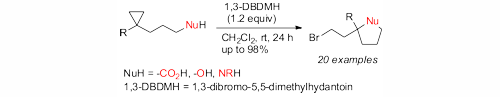
Homohalocyclization: Electrophilic Bromine-Induced Cyclizations of Cyclopropanes, C. Rösner, U. Hennecke, Org. Lett. 2015, 17, 3226-3229.© AK Hennecke 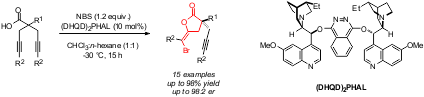
Enantioselective, Desymmetrizing Bromolactonization of Alkynes, M. Wilking, C. Mück-Lichtenfeld, C. G. Daniliuc, U. Hennecke, J. Am. Chem. Soc. 2013, 135, 8133-8136.© AK Hennecke 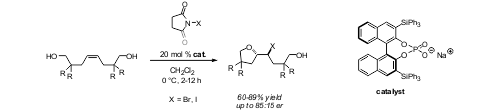
Enantioselective Haloetherification by Asymmetric Opening of meso-Halonium Ions, U. Hennecke, C. H. Müller, R. Fröhlich, Org. Lett. 2011, 13, 860-863.© AK Hennecke 
For a current review: New Catalytic Approaches towards the Enantioselective Halogenation of Alkenes, U. Hennecke, Chem. Asian J. 2012, 7, 456-465.© AK Hennecke
DNA as a construction material in catalysis and material science
We are also interested in bioorganic chemistry, especially the modification of nucleic acids and the application of DNA in nanotechnology and catalysis. This includes the development of methods for DNA modification using photochemistry or transition metal-catalyzed methodology. In the framework of the SFB 858 DNA is modified with transition metal complexes to create novel hybrid catalysts which are applied in asymmetric catalysis.
Current Publication:
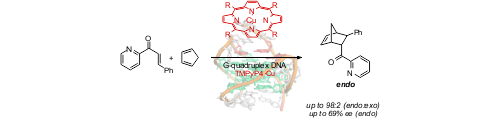
The influence of G-quadruplex structure on DNA-based asymmetric catalysis using the G-quadruplex-bound cationic porphyrin TMPyP4•Cu, M. Wilking, U. Hennecke, Org. Biomol. Chem. 2013, 11, 6940-6945.© AK Hennecke
