‘With additional heart stress, energy supply could become a problem’
Prof. Karin Busch researches the bioenergetics of cells, i.e. the processes of energy conversion that are essential for life, at the Institute of Integrative Cell Biology and Physiology. In recent studies, she has worked with Prof. Guiscard Seebohm (Faculty of Medicine) and Prof. Verónica Eisner (Pontificia Universidad Católica de Chile) in a German-Chilean team to investigate how the architecture of the mitochondria, also known as the ‘power plants’ of the cell, change in heart muscle cells with increasing age. In an interview with Christina Hoppenbrock, she provides insights into the research.
What is the significance of the structural changes in the ‘cell’s power plants’?
These changes could be a kind of “biomarker” for age-related heart decline. While the changes are associated with a reduced capacity for energy supply, they are not pathological in themselves. However, when the heart encounters additional stress, such as chronic inflammation or another disease, this limited capacity could become a serious problem. This is because the heart requires more energy to cope with such challenges.
Every cell in the body needs energy. The body’s universal energy carrier in the body is adenosine triphosphate (ATP). Mitochondria produce ATP from the breakdown of fatty acids or glucose particularly effectively compared to the ATP production through carbohydrate catabolism in glycolysis. Glycolysis takes place in a different part of the cells: the cytoplasm.
You have looked specifically at the mitochondria in heart muscle cells. What is special about them?
The different tissues and organs in the body use different energy sources. Heart muscle cells gain very little ATP via glycolysis; instead, they require the most efficient energy supply possible. They are therefore particularly dependent on having functioning mitochondria to provide them with energy.
What exactly did you investigate?
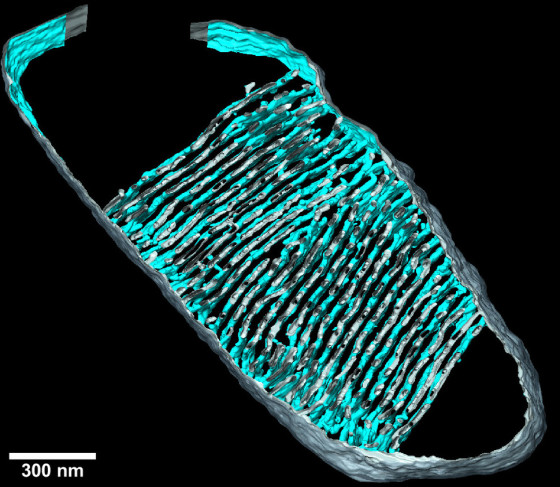
On the one hand, we looked at the architecture of the inner membrane in the mitochondria. Mitochondria are surrounded by two membranes. The inner membrane has invaginations called cristae. These are the site of oxidative phosphorylation, a series of coupled reactions that supplies ATP. We also examined in detail the protein complex that produces ATP, ATP synthase.
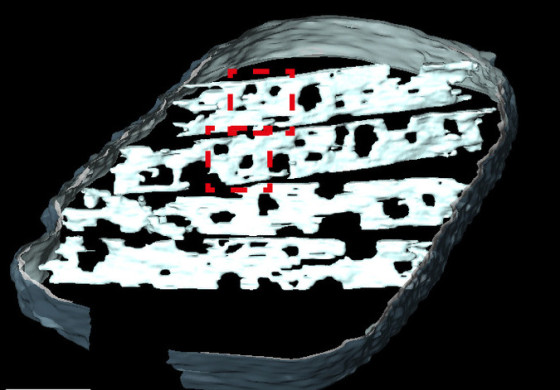
The density of cristae decreases at an early stage of the ageing process of the human heart. At this stage, however, the amount of the cristae-shaping proteins remains unchanged. Using tomographic imaging, we have shown that the cristae in older mice are less interconnected and become narrower with an increasing number of “holes”, which we refer to as “fenestrations” in the membrane. These changes were associated with a loss of the cristae-shaping protein “Opa1”. We also induced senescence in human heart muscle cells in a cell culture setting. In doing so, we observed that ATP synthase, which is normally located in the curved parts of the cristae, became more mobile. In other words, it is less firmly fixed in place. We assume that this affects its efficiency in ATP production.
Original publications
Isidora Molina-Riquelme, Gonzalo Barrientos, Leonhard Breitsprecher, +8, and Karin B. Busch, Verónica Eisner (2025): Multiscale mitochondrial cristae remodeling links Opa1 downregulation to reduced OXPHOS capacity in aged hearts. PNAS; DOI: 10.1073/pnas.2508911123
Silke Morris, Nico Marx, Gonzalo Barrientos, Isidora Molina-Riquelme, Frank Schmelter, Hugo E. Verdejo, Stefan Peischard, Guiscard Seebohm, Verónica Eisner, Karin B. Busch (2026): Disruption of ATP Synthase Spatiotemporal Organization, Ca2+ Dynamics, and Contractile Function in Senescent Cardiomyocytes. Aging Cell; DOI: 10.1111/acel.70388
