Help for stressed-out cells in a crisis
According to a team of researchers at the University of Münster, mitochondria provide unexpected help for cells in a crisis by respiring away harmful substances. A current study produced by the Institute of Biology and Biotechnology of Plants (IBBP) shows three things: that this mechanism can be triggered by reductive stress, that it protects the folding of certain proteins destined for export, and that the cell’s “powerhouse” consequently acts even more flexibly than was previously known.
“The main job that mitochondria have,” explains Prof. Markus Schwarzländer from the IBBP, “is to act as hubs of metabolism. What is surprising now is that evidently they are also able to take care of an excess of specific compounds, so-called thiols, which could otherwise lead to damage at other places in the cell.” To support this a special crisis programme is triggered, the ‘ANAC017 signalling pathway’. “The ‘alternative oxidase’ protein then ensures there is a higher respiratory capacity in the mitochondria in plants,” says Schwarzländer. This process was jointly discovered some years ago by two teams of researchers from Australia and Belgium. What is new now is the discovery that it can be triggered through reductive stress and can serve to protect protein folding in the endoplasmic reticulum (ER), i.e. the export system for proteins in the cell. Something that is also new – and unexpected – is that the mitochondria manage to respire away thiols at a high rate. “This contradicts current thinking,” Schwarzländer concedes, “which is why we used several different methods in order to be able to validate our observations independently and rule out any possible errors.” To this end, the team developed a new method for dynamically investigating the energisation of mitochondria.

Background:
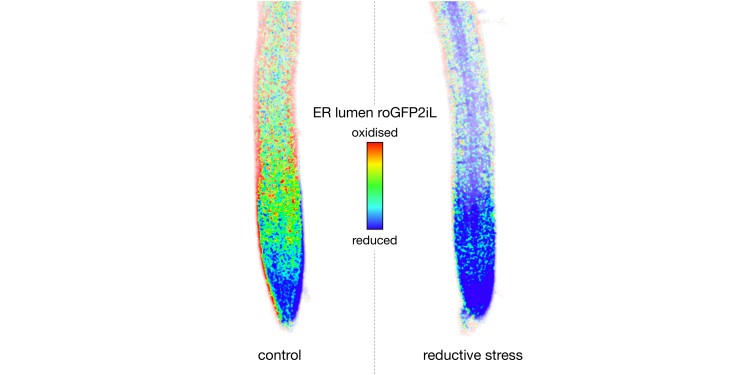
All life consists of cells. All cells need proteins, which have to be folded very precisely to be able to fulfil their function. Proteins which are secreted from animals, fungi and plants – or which contribute to environmental interaction on their surface – have to be stabilised by means of so-called disulfide bridges. One example of this in humans is insulin. In general, though, countless vital receptor and signalling proteins only function with the correct links via disulfide bridges. These are established at a very precise place in the cell, the so-called endoplasmic reticulum (ER). For this purpose, sulfur atoms from each of two thiol groups of the amino-acid cysteine in the interior of the ER are oxidised and linked covalently.
If, however, more disulfide bridges are suddenly needed, or if they are broken up by a stress factor or by certain chemical substances, this is problematic for the cell. Wrongly folded proteins can cause extensive damage, even death. It is for this reason that the cell reacts with special crisis programmes. These well-examined programmes support protein-folding in the interior of the ER – for example, by providing additional capacity in the oxidisation machinery. The novel finding in this study is the discovery that, when a crisis arises, the ER receives support from another area of the cell.
Methodology
Decisive parts of the current study are based on ‘in vivo bio-sensor technology’. The innovative use of optogenetic bio-sensor technology is a core competence of the IBBP Münster team, with synthetic proteins serving as measuring sensors. They are genetically encoded, so that it is the plants themselves which produce the sensors. In a living plant cell, these then provide ‘live’ information on the metabolic status. Such sensors can be introduced into individual parts of the cell for measuring purposes, which means that the organisation within the cell is preserved. As this requires quantitative microscopy in the living organism, the Institute successfully submitted an application for large-scale equipment in 2019 – specifically, for a high-resolution confocal microscope. This equipment is operated as part of the ‘Imaging Network’ at Münster University.
All the work was carried out at the Universities of Münster and Bonn. The project began in a Research Training Group in Bonn, funded by the German Research Association, and continued in Bonn and Münster after the research group relocated in 2017. Today, some of the co-authors work at other institutes such as the Max Planck Institute in Cologne, Kaiserslautern Technical University and the University of Milan. Lead author Philippe Fuchs today works in the field of sustainable crop protection at a company in Switzerland, but he played a decisive role in the work from its inception to its publication.
Funding
The study was funded by the German Research Foundation (DFG).
Original publication
Fuchs P, Bohle F, Lichtenauer S, Ugalde JM, Feitosa Araujo E, Mansuroglu B, Ruberti C, Wagner S, Müller-Schüssele SJ, Meyer AJ, Schwarzländer M (2022) Reductive stress triggers NAC17-mediated retrograde signaling to safeguard the ER by boosting mitochondrial respiratory capacity. Plant Cell.
DOI: 10.1093/plcell/koac017
