New mechanism of force transduction in muscle cells discovered
The ability of cells to sense and respond to their mechanical environment is critical for many cellular processes but the molecular mechanisms underlying cellular mechanosensitivity are still unclear. Researchers at the University of Münster have now discovered how the muscle-specific adhesion molecule metavinculin modulates mechanical force transduction on the molecular level. The research results have just been published in the journal "Nature Communications".
Background and methodology
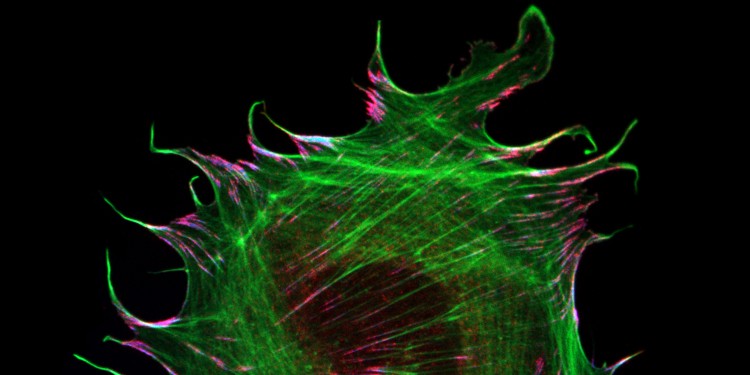
The interaction of cells with their environment is mediated by specialized adhesion structures, which transduce mechanical forces inwards and out of cells. As cellular adhesions consist of hundreds of different proteins, it is still unclear how the mechanical information is transmitted on the molecular level. To study these processes in more detail, the Grashoff laboratory at the WWU Münster develops biosensors that allow the detection of piconewton-scale forces propagated across individual molecules in cells. In their most recent study, the authors applied their microscopy-based technique to the adhesion protein metavinculin, which is expressed in muscle cells and associated with cardiomyopathy, a heart muscle disease.
By analyzing a range of genetically modified cells, the authors demonstrate that the presence of metavinculin changes how mechanical forces are transduced in cell adhesion complexes. “Our data indicate that metavinculin could function as a molecular dampener, helping to resist high peak forces observed in muscle tissues“, explains Prof. Dr. Carsten Grashoff, principal investigator of the study. “This is a very interesting example of how the presence of a single protein can change the way mechanical information is processed in cells.”
Surprisingly, the authors did not observe any indications of cardiomyopathy in mice lacking metavinculin. This suggests that the pathophysiological role of metavinculin is more complex than previously assumed.
Funding
The research work was funded by the German Research Foundation (DFG).
Original publication
Verena Kanoldt, Carleen Kluger, Christiane Barz, Anna-Lena Schweizer, Deepak Ramanujam, Lukas Windgasse, Stefan Engelhardt, Anna Chrostek-Grashoff, and Carsten Grashoff. Metavinculin modulates force transduction in cell adhesion sites. "Nature Communications". DOI : 10.1038/s41467-020-20125-z
