





The newly established Institute of Integrative Cell Biology and Physiology (IIZP) integrates the former Institute of Molecular Cell Biology and the Institute of Zoophysiology.
Our new institute embodies the close connection between physiological and cell biological processes that cannot be fully understood in isolation. At the IIZP, our mission is to explore the complex structures and physiological processes in animal cells and whole organisms across a range of time and length scales. Following this approach, our aim is to develop a comprehensive understanding of the molecular, cellular and biophysical principles of developmentally, physiologically and pathologically relevant processes.
The research activities at the IIZP integrate experiments on individual molecules, isolated cells, tissues and living organisms. We are using a wide range of model systems that include cell cultures, amoebae, crustaceans, nematodes, fruit flies as well as mouse models. For our integrative approach, we combine biochemical and genetic technologies with high-resolution, quantitative microscopy approaches.
Please visit our research groups for more information.
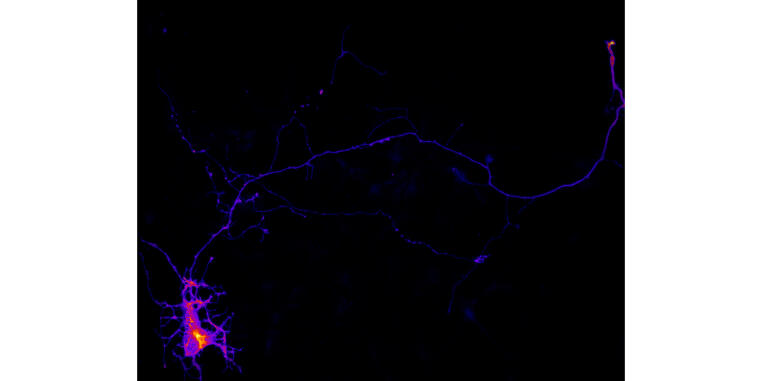
During their differentiation, neurons establish a highly polarized morphology by extending a single axon and multiple dendrites. This process depends on a combination of positive and negative feedback signals that promote axon formation and prevent immature dendrites from developing into axons. Our study shows that the generation of the phospholipid phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) acts as a negative feedback signal that regulates the activity of the GTPase Rap1. Impairing the function of the PI(4,5)P2 generating lipid kinase Pip5k1γ or manipulating membrane tension by osmotic shock results in a hyper-activation of Rap1, which induces the formation of supernumerary axons. These results show that PI(4,5)P2-dependent membrane properties limit the activity of Rap1 to ensure the extension of a single axon.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft.
Original publication
Di Meo, D., Kundu, T. Ravindran, P., Shah, B, Püschel, A.W. (2024). Pip5k1γ regulates axon formation by limiting Rap1 activity. Life Sci Alliance 7: e202302383. doi: 10.26508/lsa.202302383.
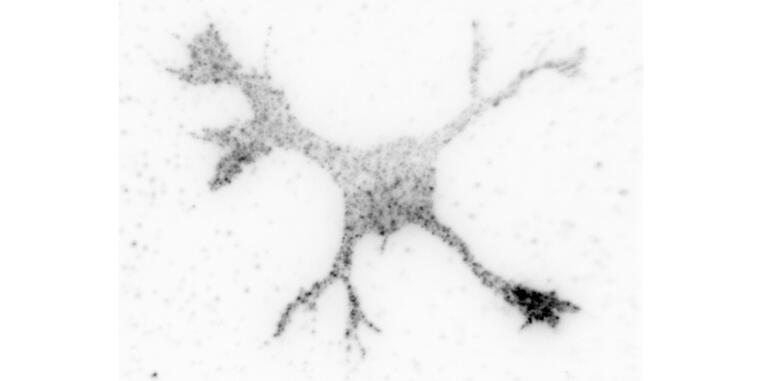
The formation of an axon and its rapid extension require the expansion of the plasma membrane by the exocytosis of specialized vesicles. These vesicles are tethered at the membrane by the exocyst complex prior to their fusion. The highly conserved GTPase Cdc42 promotes exocytosis through the exocyst complex in budding yeast and Drosophila. In mammals, this function is performed by the closely related GTPase TC10 while mammalian Cdc42 was reported not to interact with the exocyst. Two Cdc42 isoforms are expressed in the mammalian brain that are generated by alternative splicing and differ only in their C-terminal 10 amino acids. The publication by Ravindran and Püschel shows that the brain-specific - but not the ubiquitous Cdc42 isoform - can interact with Exo70. The brain-specific Cdc42 isoform promotes exocytosis in the growth cone during neuronal polarization downstream of its activator Arhgef7. Thus, the function of Cdc42 in regulating exocytosis is conserved also in mammals but specific to one isoform.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft.
Original publication
Ravindran P, Püschel AW (2022). An isoform-specific function of Cdc42 in regulating mammalian Exo70 during axon formation. Life Sci Alliance: e202201722. doi: 10.26508/lsa.202201722.
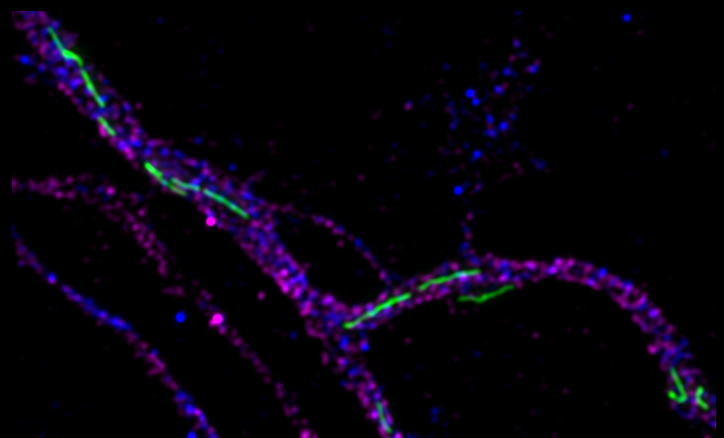
Neurons are highly polarized cells that display characteristic differences in the organization of their organelles in axons and dendrites. The kinases SadA and SadB (SadA/B, also called Brsk2 and Brsk1) promote the formation of distinct axonal and dendritic extensions during the development of cortical and hippocampal neurons. The work of Danila Di Meo, Priyadarshini Ravindran, Pratibha Dhumale, Tanmay Sadhanasatish and Andreas Püschel shows that the specific dynamics of axonal mitochondria depends on these kinases. They function as regulators of mitochondrial dynamics through the phosphorylation of the microtubule-binding protein Tau. Suppression of SadA/B in cortical neurons disrupts the balance of mitochondrial fission and fusion and induces the elongation of mitochondria. SadA/B-deficient neurons show an accumulation of hyper-fused mitochondria and the activation of the integrated stress response. SadA/B regulate the interaction of Tau with actin filaments that are essential for the Drp1-mediated fission of mitochondria. The elongation of mitochondria after a loss of SadA/B results from an excessive stabilization of actin filaments and reduction of Drp1 recruitment to mitochondria. The normal dynamics of axonal mitochondria could be restored by mild actin destabilization. These results identify a new pathway that regulates mitochondrial dynamics through Tau.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft.
Original publication
Di Meo, D., Ravindran, P., Sadhanasatish, T., Dhumale, P., Püschel, A.W. (2021). The balance of mitochondrial fission and fusion in cortical axons depends on the kinases SadA and SadB. Cell Rep. 37, 110141. doi: 10.1016/j.celrep.2021.110141.
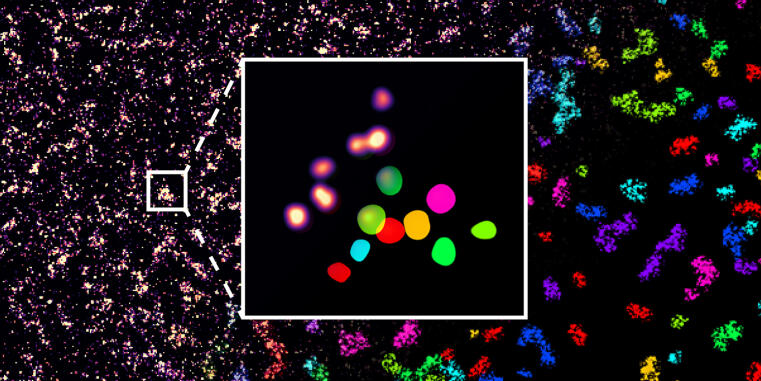
WWU researchers optimize super-resolution microscopy application for single molecule detection / Study published as cover story in ChemBioChem
The development of super-resolution microscopy, which was awarded the Nobel Prize in 2014, allows the analysis of cell biological processes with a precision of a few nanometers, making it even possible to differentiate between individual molecules within cells. One limitation of virtually all single-molecule resolved super-resolution approaches, however, is that the target molecules have to be genetically modified to allow precise measurements. Researchers at the WWU Münster have now optimized a protein labeling process so that native, unmodified proteins can be visualized and quantified with molecular resolution in their natural environment.
Background and method
High-resolution microscopy is typically based upon a procedure, in which a transient interaction between a fluorescent probe and the target molecule produces an isolated blinking signal that can be used to calculate its precise localization. For this purpose, however, the molecule of interest is usually genetically modified. For example, DNA-PAINT is based on the fact that a DNA binding site is attached to the target protein, to which a complementary fluorescent DNA strand can bind for detection. Other high-resolution microscopy methods use modifications by fluorescent proteins. Since such genetic modifications are undesirable in many applications and sometimes not even possible, new methods for the detection of unmodified, endogenous proteins are needed.
Lisa Fischer, PhD student in Prof. Carsten Grashoff's group, has therefore developed a procedure called Direct Peptide-PAINT, with which a central cell adhesion protein, Talin, can be labeled by a fluorescent interaction peptide. The first application did not only reveal the molecular distribution of this molecule in differentiating stem cells, it also allowed the first visualization of individual Talin proteins in tissue sections. The new method should therefore allow the investigation of adhesion processes under pathophysiological relevant conditions. The researchers expect that the new technique will be useful to obtain important, molecular insights into disorders that are based on dysfunctional cell adhesion.
Funding
The work was funded by the Deutsche Forschungsgemeinschaft (DFG) and the Human Frontier Science Program.
Original publication
Lisa S. Fischer, Thomas Schlichthaerle, Anna Chrostek-Grashoff, and Carsten Grashoff. Peptide-PAINT Enables Investigation of Endogenous Talin with Molecular Scale Resolution in Cells and Tissues. Chembiochem. DOI: 10.1002/cbic.202100301