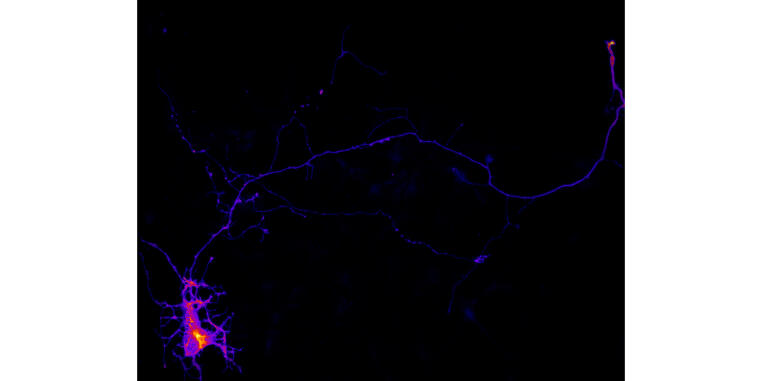
New study reveals role of phospholipids and membrane tension in axon formation
During their differentiation, neurons establish a highly polarized morphology by extending a single axon and multiple dendrites. This process depends on a combination of positive and negative feedback signals that promote axon formation and prevent immature dendrites from developing into axons. Our study shows that the generation of the phospholipid phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) acts as a negative feedback signal that regulates the activity of the GTPase Rap1. Impairing the function of the PI(4,5)P2 generating lipid kinase Pip5k1γ or manipulating membrane tension by osmotic shock results in a hyper-activation of Rap1, which induces the formation of supernumerary axons. These results show that PI(4,5)P2-dependent membrane properties limit the activity of Rap1 to ensure the extension of a single axon.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft.
Original publication
Di Meo, D., Kundu, T. Ravindran, P., Shah, B, Püschel, A.W. (2024). Pip5k1γ regulates axon formation by limiting Rap1 activity. Life Sci Alliance 7: e202302383. doi: 10.26508/lsa.202302383.
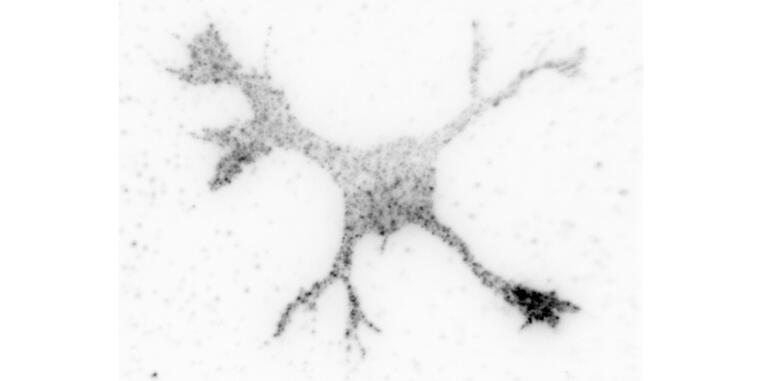
New function during axon formation identified for the GTPase Cdc42
The formation of an axon and its rapid extension require the expansion of the plasma membrane by the exocytosis of specialized vesicles. These vesicles are tethered at the membrane by the exocyst complex prior to their fusion. The highly conserved GTPase Cdc42 promotes exocytosis through the exocyst complex in budding yeast and Drosophila. In mammals, this function is performed by the closely related GTPase TC10 while mammalian Cdc42 was reported not to interact with the exocyst. Two Cdc42 isoforms are expressed in the mammalian brain that are generated by alternative splicing and differ only in their C-terminal 10 amino acids. The publication by Ravindran and Püschel shows that the brain-specific - but not the ubiquitous Cdc42 isoform - can interact with Exo70. The brain-specific Cdc42 isoform promotes exocytosis in the growth cone during neuronal polarization downstream of its activator Arhgef7. Thus, the function of Cdc42 in regulating exocytosis is conserved also in mammals but specific to one isoform.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft.
Original publication
Ravindran P, Püschel AW (2022). An isoform-specific function of Cdc42 in regulating mammalian Exo70 during axon formation. Life Sci Alliance: e202201722. doi: 10.26508/lsa.202201722.
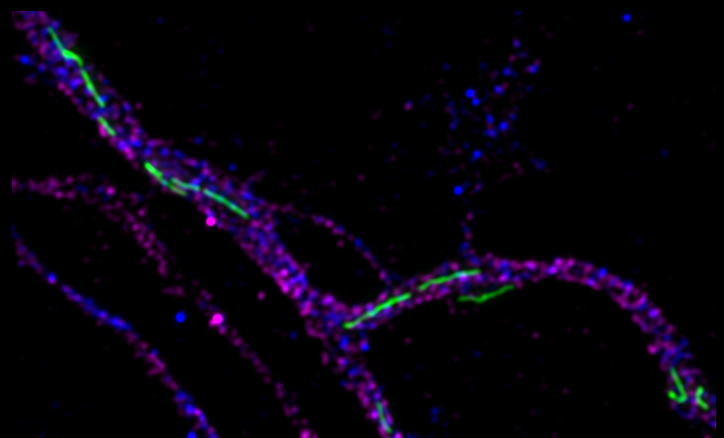
New pathway identified that regulates axonal mitochondria
Neurons are highly polarized cells that display characteristic differences in the organization of their organelles in axons and dendrites. The kinases SadA and SadB (SadA/B, also called Brsk2 and Brsk1) promote the formation of distinct axonal and dendritic extensions during the development of cortical and hippocampal neurons. The work of Danila Di Meo, Priyadarshini Ravindran, Pratibha Dhumale, Tanmay Sadhanasatish and Andreas Püschel shows that the specific dynamics of axonal mitochondria depends on these kinases. They function as regulators of mitochondrial dynamics through the phosphorylation of the microtubule-binding protein Tau. Suppression of SadA/B in cortical neurons disrupts the balance of mitochondrial fission and fusion and induces the elongation of mitochondria. SadA/B-deficient neurons show an accumulation of hyper-fused mitochondria and the activation of the integrated stress response. SadA/B regulate the interaction of Tau with actin filaments that are essential for the Drp1-mediated fission of mitochondria. The elongation of mitochondria after a loss of SadA/B results from an excessive stabilization of actin filaments and reduction of Drp1 recruitment to mitochondria. The normal dynamics of axonal mitochondria could be restored by mild actin destabilization. These results identify a new pathway that regulates mitochondrial dynamics through Tau.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft.
Original publication
Di Meo, D., Ravindran, P., Sadhanasatish, T., Dhumale, P., Püschel, A.W. (2021). The balance of mitochondrial fission and fusion in cortical axons depends on the kinases SadA and SadB. Cell Rep. 37, 110141. doi: 10.1016/j.celrep.2021.110141.
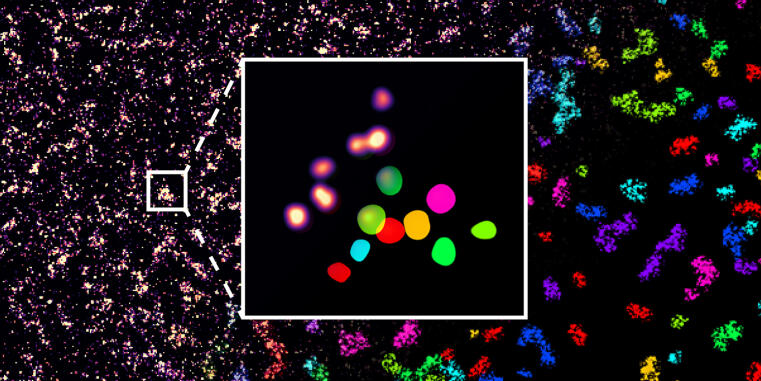
Optimized microscopy method enables single molecule detection under native conditions
WWU researchers optimize super-resolution microscopy application for single molecule detection / Study published as cover story in ChemBioChem
The development of super-resolution microscopy, which was awarded the Nobel Prize in 2014, allows the analysis of cell biological processes with a precision of a few nanometers, making it even possible to differentiate between individual molecules within cells. One limitation of virtually all single-molecule resolved super-resolution approaches, however, is that the target molecules have to be genetically modified to allow precise measurements. Researchers at the WWU Münster have now optimized a protein labeling process so that native, unmodified proteins can be visualized and quantified with molecular resolution in their natural environment.
Background and method
High-resolution microscopy is typically based upon a procedure, in which a transient interaction between a fluorescent probe and the target molecule produces an isolated blinking signal that can be used to calculate its precise localization. For this purpose, however, the molecule of interest is usually genetically modified. For example, DNA-PAINT is based on the fact that a DNA binding site is attached to the target protein, to which a complementary fluorescent DNA strand can bind for detection. Other high-resolution microscopy methods use modifications by fluorescent proteins. Since such genetic modifications are undesirable in many applications and sometimes not even possible, new methods for the detection of unmodified, endogenous proteins are needed.
Lisa Fischer, PhD student in Prof. Carsten Grashoff's group, has therefore developed a procedure called Direct Peptide-PAINT, with which a central cell adhesion protein, Talin, can be labeled by a fluorescent interaction peptide. The first application did not only reveal the molecular distribution of this molecule in differentiating stem cells, it also allowed the first visualization of individual Talin proteins in tissue sections. The new method should therefore allow the investigation of adhesion processes under pathophysiological relevant conditions. The researchers expect that the new technique will be useful to obtain important, molecular insights into disorders that are based on dysfunctional cell adhesion.
Funding
The work was funded by the Deutsche Forschungsgemeinschaft (DFG) and the Human Frontier Science Program.
Original publication
Lisa S. Fischer, Thomas Schlichthaerle, Anna Chrostek-Grashoff, and Carsten Grashoff. Peptide-PAINT Enables Investigation of Endogenous Talin with Molecular Scale Resolution in Cells and Tissues. DOI: 10.1002/cbic.202100301

New microscopy analysis allows discovery of central adhesion complex
WWU researchers develop a new method for quantitative single-molecule colocalization analysis /Study published in Nature Communications
Cells of organisms are organized in subcellular compartments that consist of many individual molecules. How these single proteins are organized on the molecular level remains unclear, because suitable analytical methods are still missing. Researchers at the University of Münster together with colleagues from the Max Planck Institute of Biochemistry (Munich) have established a new technique that enables quantifying molecular densities and nanoscale organizations of individual proteins inside cells. The first application of this approach reveals a complex of three adhesion proteins that appears to be crucial for the ability of cells to adhere to the surrounding tissue. The research results have just been published in the journal Nature Communications.more
Funding
The research work was funded by the German Research Foundation (DFG).
Original publication
Verena Kanoldt, Carleen Kluger, Christiane Barz, Anna-Lena Schweizer, Deepak Ramanujam, Lukas Windgasse, Stefan Engelhardt, Anna Chrostek-Grashoff, and Carsten Grashoff. Metavinculin modulates force transduction in cell adhesion sites. Nature Communications. DOI : 10.1038/s41467-020-20125-z.
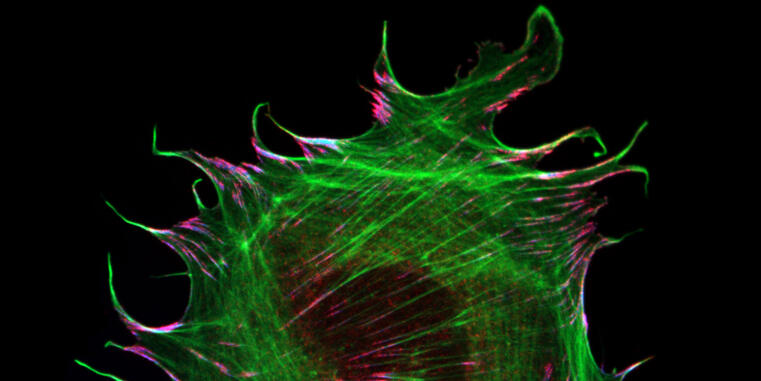
New mechanism of force transduction in muscle cells discovered
WWU researchers reveal mechanobiological function of muscle-specific adhesion protein / Study published in Nature Communications
The ability of cells to sense and respond to their mechanical environment is critical for many cellular processes but the molecular mechanisms underlying cellular mechanosensitivity are still unclear. Researchers at the University of Münster have now discovered how the muscle-specific adhesion molecule metavinculin modulates mechanical force transduction on the molecular level. The research results have just been published in the journal Nature Communications.
Background and methodology
The interaction of cells with their environment is mediated by specialized adhesion structures, which transduce mechanical forces inwards and out of cells. As cellular adhesions consist of hundreds of different proteins, it is still unclear how the mechanical information is transmitted on the molecular level. To study these processes in more detail, the Grashoff laboratory at the WWU Münster develops biosensors that allow the detection of piconewton-scale forces propagated across individual molecules in cells. In their most recent study, the authors applied their microscopy-based technique to the adhesion protein metavinculin, which is expressed in muscle cells and associated with cardiomyopathy, a heart muscle disease.
By analyzing a range of genetically modified cells, the authors demonstrate that the presence of metavinculin changes how mechanical forces are transduced in cell adhesion complexes. “Our data indicate that metavinculin could function as a molecular dampener, helping to resist high peak forces observed in muscle tissues“, explains Prof. Dr. Carsten Grashoff, principal investigator of the study. “This is a very interesting example of how the presence of a single protein can change the way mechanical information is processed in cells.”
Surprisingly, the authors did not observe any indications of cardiomyopathy in mice lacking metavinculin. This suggests that the pathophysiological role of metavinculin is more complex than previously assumed.more
Funding
The research work was funded by the German Research Foundation (DFG).
Original publication
Verena Kanoldt, Carleen Kluger, Christiane Barz, Anna-Lena Schweizer, Deepak Ramanujam, Lukas Windgasse, Stefan Engelhardt, Anna Chrostek-Grashoff, and Carsten Grashoff. Metavinculin modulates force transduction in cell adhesion sites. Nature Communications. DOI : 10.1038/s41467-020-20125-z.

Myosins: A Superfamily of Molecular Motors
A comprehensive account was published of the current understanding of myosins, actin-based molecular motors, that contains a chapter on the current knowledge of class IX myosins that was contributed by the Bähler group. Class IX myosins demonstrate not only unique motor properties, but simultaneously serve as negative regulators of an important signaling pathway that controls various cellular processes such as e.g. cell morphology and cell migration.

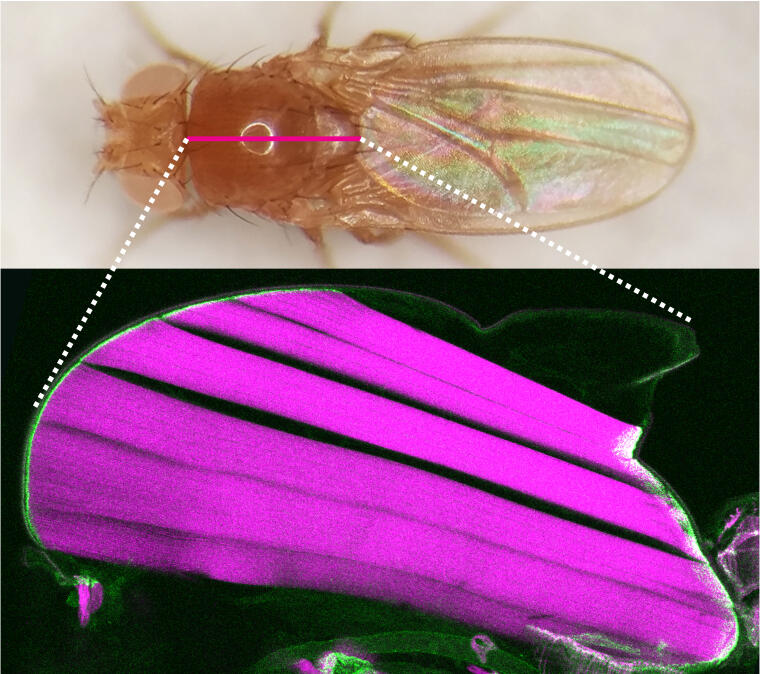
How do muscle and tendon connections last a lifetime?
Cell biologists show in fruit flies how a protein controls mechanical stress on muscle-tendon attachments.
Muscles are connected to tendons to power animal movements such as running, swimming or flying. Forces are produced by myofibrils, contractile chains of actin and myosin, which are pulling on muscle-tendon connections called attachments. During animal development, these muscle-tendon attachments must be established such that they resist high mechanical forces for the entire life of the animal. How the individual protein molecules that build the attachments ‘feel’ the mechanical forces inside an intact muscle can now be measured with modern cell and developmental biology techniques. An interdisciplinary team lead by Frank Schnorrer and Carsten Grashoff at the Developmental Biology Institute of CNRS & Aix Marseille University, the Max Planck Institute of Biochemistry in Munich and the Institute for Molecular Cell Biology at University of Münster has now been able to quantify the mechanical forces transmitted by a key attachment protein called Talin during the development of muscle attachments. Sandra Lemke, a PhD student in the Schnorrer group, used the flight muscles of the fruit fly Drosophila for these molecular force measurements and found that a surprisingly small proportion of Talin molecules experiences detectable forces at developing muscle-tendon attachments. She found that muscles deal with the increasing tissue forces by recruiting an high number of Talin molecules to attachments. This way, many Talin molecules can dynamically share the high peak forces produced during muscle contractions, for example while flying. This mechanical adaptation concept ensures that muscle-tendon connections can last for life. These new results have just been published in PLoS Biology. (more)
Original publication
S. Lemke et al. (2019): A small proportion of Talin molecules transmit forces at developing muscle attachments in vivo. PLOS Biology; DOI: 10.1371/journal.pbio.3000057
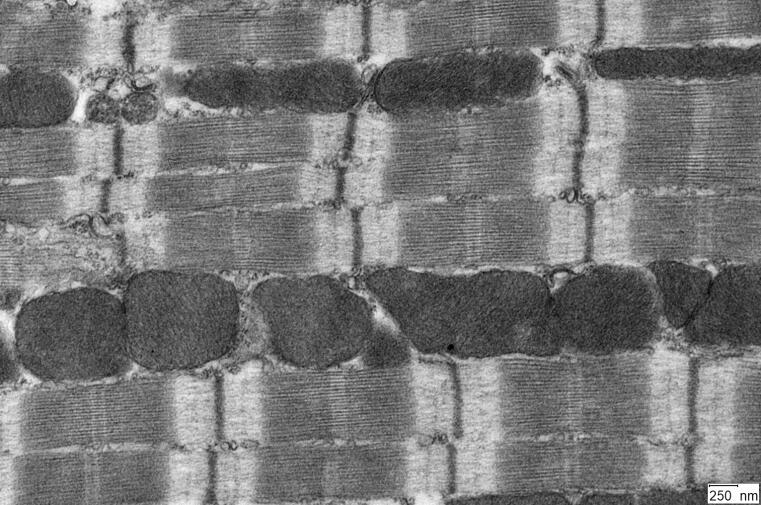
Discovery of a new heart muscle component
Researchers at Münster University have identified a motor protein that is important for the contractile units of heart muscle.
In order for the heart to work properly, it must exert muscular force. This involves the coordinated contraction of numerous sarcomeres, the smallest contractile units of heart muscle. Muscle contraction is brought about by the activity of conventional motor proteins, which pull on thin filaments to shorten sarcomeres. Together with researchers from Toronto (Canada) and Leiden (the Netherlands), scientists from the University of Münster have now found out more about the function of a specific unconventional motor protein, myosin 18A (Myo18A): they have discovered a new variant of the protein that appears to be responsible for the assembly and mechanical stability of sarcomeres in the heart. The results could help scientists better understand how sarcomeres are formed and regulated. The study has been published in “The Journal of Biological Chemistry”, where it was selected as an “Editors’ Pick” and identified as a research highlight. (more)
Funding
The study received financial support from the German Research Foundation, from the Cells-in-Motion Cluster of Excellence at Münster University, and from the Canadian research organizations “Genome Canada” and “Ontario Genomics”.
Original publication
Horsthemke M. et al. (2019): A novel isoform of myosin 18A (Myo18Aγ) is an essential sarcomeric protein in mouse heart. Journal of Biological Chemistry; DOI: 10.1074/jbc.RA118.004560
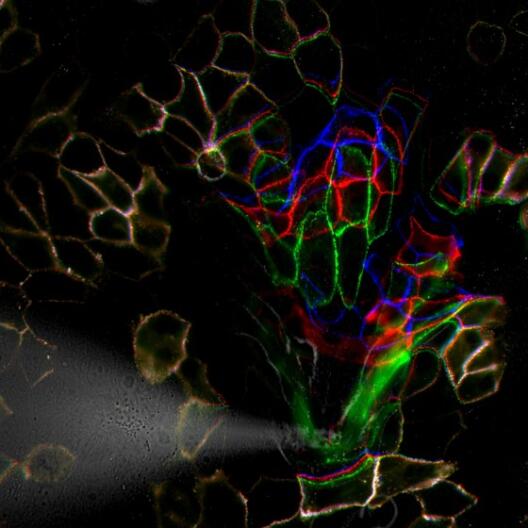
How skin cells protect themselves against stress
Cell biologists at Münster University develop new method for measuring how mechanical forces in cells are processed.
The skin is our largest organ, and, among other things, it provides protection against mechanical impacts. To ensure this protection, skin cells have to be connected to one another especially closely. Exactly how this mechanical stability is provided on the molecular level was unclear for a long time. Researchers in the team led by Prof. Carsten Grashoff from the Institute of Molecular Cell Biology at the University of Münster and the Max Planck Institute of Biochemistry have been collaborating with colleagues at Stanford University in the USA, and they are now able to demonstrate how mechanical stress on specialized adhesion points, so-called desmosomes, is processed. They designed a mini-measuring device, which can determine forces along individual components of the desmosomes. In the study, published in “Nature Communications”, they show how mechanical forces propagate along these structures. (more)
Original publication
Price, J. A.; Cost, A. L.; Ungewiß, H.; Waschke, J.; Dunn, A. R.; Grashoff, C. Mechanical loading of desmosomes depends on the magnitude and orientation of external stress. Nature Communications (2018). DOI: 10.1038/s41467-018-07523-0.
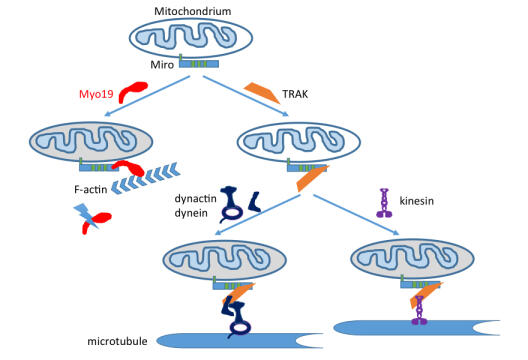
How tractor units are connected to their freight and reach their delivery point in cells
AG Bähler publishes new research findings.
Mitochondria are the power plants of the cell. They possess their own genetic information and therefore cannot be newly created and must be evenly distributed to the daughter cells during cell division. Mitochondria can be transported in cells along two different transport pathways, the cytoskeletal fibers microtubules and actin filaments. Work by the Bähler group has now identified the attachment site on mitochondria for the motor molecule myosin XIX (Myo19), which mediates mitochondrial transport along actin filaments. This attachment site stabilizes the motor molecule Myo19, and when it is absent, the motor molecule Myo19 is disposed of. Interestingly, this attachment site also serves indirectly via a connector for the attachment of the motors responsible for bidirectional transport of mitochondria along microtubules. There is therefore a competition among the three motors for the same attachment site on the mitochondria and, accordingly, their correct distribution and delivery.
Original publication
Oeding SJ, Majstrowicz K, Hu XP, Schwarz V, Freitag A, Honnert U, Nikolaus P, Bähler M.
J Cell Sci. 2018 Sep 10;131(17). pii: jcs219469. doi: 10.1242/jcs.219469.

Carsten Grashoff awarded the BINDER Innovation Prize 2018
The German Society for Cell Biology acknowledges each year outstanding cell biological research with the BINDER Innovation Prize. In 2018, the recognition goes to Prof. Dr. Carsten Grashoff for the development and application of methods allowing the quantitative analysis of mechanical forces in cells. The technique permits the investigation of mechanical processes under physiological conditions and has become a valuable tool to study the mechanobiology of cells and tissues. The prize giving ceremony will be held at the International Meeting of the German Society for Cell Biology in Leipzig on September 17, 2018.
The ability to generate, sense, and respond to mechanical forces is a property inherent to all cells. However, it has remained unclear how mechanical signals are processed on the molecular level because suitable methods to quantify forces across individual proteins in living cells were missing. Carsten Grashoff and his research team have developed and optimized techniques that overcome this limitation by generating genetically-encoded calibrated biosensors, which can detect extremely small mechanical forces as little as a few piconewton. When inserted into individual proteins and expressed in cells, these probes allow spatially and temporally resolved molecular force measurements using quantitative microscopy techniques. Initial studies of the Grashoff lab primarily focused on the analysis of cell adhesion mechanics leading to the identification of mechanical linkages that are crucial for proper adhesion and rigidity sensing of cells. By now, the so-called tension sensor technique has been also applied in many other research laboratories to investigate biomechanical processes underlying cellular force transduction.
Carsten Grashoff studied Applied Science at the University of Freiberg and earned his PhD degree at the Max Planck Institute of Biochemistry in 2007. For his postdoctoral work, he moved to the University of Virginia (USA) and in 2011 he returned to the Max Planck Institute of Biochemistry as an independent research group leader. In 2018, Carsten Grashoff was appointed as a Professor at the University of Münster.
The German Society for Cell Biology represents and promotes the interests of cell biology as a multidisciplinary, comprehensive science in Germany. The BINDER Innovation Prize acknowledges outstanding research in the field of cell biology since 1998. The award is endowed with 4,000 Euro sponsored by BINDER GmbH.

Using spider web proteins to measure the smallest forces in cells
In his inaugural lecture on Friday, June 22, Prof. Dr. Carsten Grashoff from the Institute of Molecular Cell Biology at WWU will focus on molecular force transmission in cells.
The lecture will begin at 4:15 p.m. in lecture hall ZH, Badestraße 9. All interested parties are invited to the event. Admission is free.

"Human Frontier Science Program" funds two projects involving Münster University researchers
In the selection phase for 2018, the prestigious “Program Grant” research award given by the international “Human Frontier Science Program” goes to two members of the Department of Biology at the University of Münster – to bioinformatics specialist Prof. Erich Bornberg-Bauer and cell biologist Prof. Karin Busch. (more)

Appointment
Dr. Carsten Grashoff of the Max Planck Institute of Biochemistry, Martinsried, has been appointed University Professor for the subject "Quantitative Cell Biology" at the Institute of Molecular Cell Biology, effective January 15, 2018.


