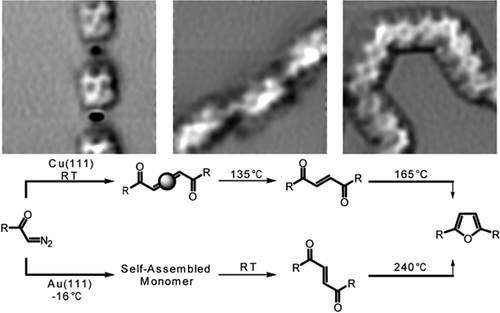
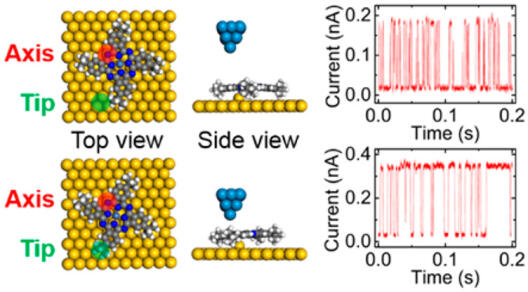
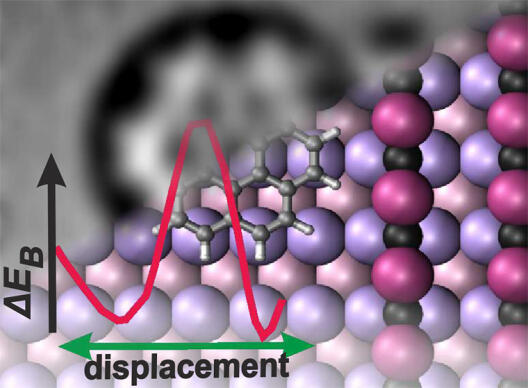
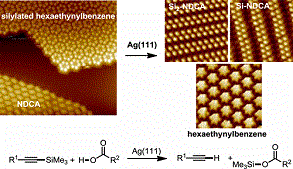
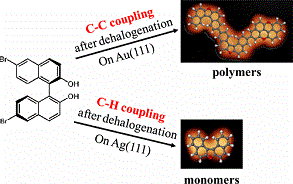
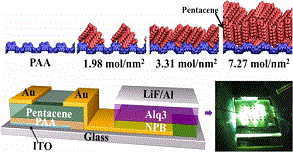
Selected recent publications
Book
Soft Matters for Catalysts
Edited by Qingmin Ji and H. Fuchs
2019, ISBN: 9789814774666
Jenny Stanford Publishing Pte. Ltd., Singapore
Chapter 7: Catalytic reactions on Solid Surfaces
By Huihui Kong, Xinbang Liu, and Harald Fuchs
Chapter 8: Soft Matters for Duture Catalysts: A Perspective
By Qingmin Ji, Harald Fuchs, and Katsuhiko Arigo

With the increasing demand for optimization of energy storage, maintenance of the environment, and effective production, control on nanostructures of catalysts and optimization of their organization have become key to achieving high efficiency and specificity in energy and material conversion systems. This book emphasizes and summarizes the novel design of soft matters (molecules, polymers, assembled motifs, etc.) for nanocatalysts and nanocatalyst supports. The diversity or specialty of soft matters offers a new perspective and great promise for the development of new nanocatalytic systems for future requirements. Soft matters can provide a simple and well-defined space for the discovery of new catalysts.
This book covers nonmetallic organocatalysts, organometallic compounds, dendrimers, ionic liquids, enzymes, polymers, various organized nanoarchitectures for supporting catalysts, and molecular dynamics in catalytic surface reactions. It gives readers a complete picture of the catalysis systems based on soft matters and is a useful reference for advanced undergraduate- and graduate-level students and researchers in chemistry, biology, materials science, nanoscience, polymer science, and catalysis.