Selected recent publications
Book
Soft Matters for Catalysts
Edited by Qingmin Ji and

With the increasing demand for optimization of energy storage, maintenance of the environment, and effective production, control on nanostructures of catalysts and optimization of their organization have become key to achieving high efficiency and specificity in energy and material conversion systems. This book emphasizes and summarizes the novel design of soft matters (molecules, polymers, assembled motifs, etc.) for nanocatalysts and nanocatalyst supports. The diversity or specialty of soft matters offers a new perspective and great promise for the development of new nanocatalytic systems for future requirements. Soft matters can provide a simple and well-defined space for the discovery of new catalysts.
This book covers nonmetallic organocatalysts, organometallic compounds, dendrimers, ionic liquids, enzymes, polymers, various organized nanoarchitectures for supporting catalysts, and molecular dynamics in catalytic surface reactions. It gives readers a complete picture of the catalysis systems based on soft matters and is a useful reference for advanced undergraduate- and graduate-level students and researchers in chemistry, biology, materials science, nanoscience, polymer science, and catalysis.
Deyang Ji, Tao Li, and
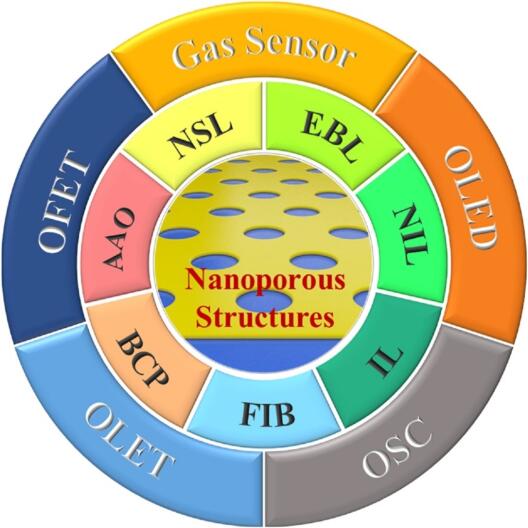
Organic electronic devices have received intensive interests from both academia and industry due to their potential for low cost, large-area, and solution-processed alternative to conventional inorganic devices. The integration of nanoporous structures into organic electronics endows devices with unique capabilities for enhanced performance and promising caopacity for various applications. Recent advances in patterning abd applications of nanoporous structures have greatly promoted the progress of organic electronics. This review summaries the current research activities on the fabrication methiodologies of nanoporous structures and their achievements in organic electronic devices. Finally, an outlook of future research directions and challenges in this area is presented.
A. Bakker, A. Timmer, E. Kolodzeiski, M. Freitag, H.-Y. Gao, H. Mönig, S. Amirjalayer, F. Glorius, H. Fuchs
Elucidating the binding modes of N-heterocyclic carbenes on a gold surface
J. Am. Chem. Soc., 2018, 140 (38), pp 11889–11892
Abstract

Tuning the binding mode of N-heterocyclic carbenes (NHCs) on metal surfaces is crucial for the development of new functional materials. To understand the impact of alkyl sidegroups on the formation of NHCs species at the Au(111) surface, we combined scanning tunneling microscopy (STM), X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) calculations. We reveal two significantly different binding modes depending on the alkyl length. In case of the short alkyl-substituent an up-standing configuration with one Au adatom is preferred, whereas the longer alkyl groups result exclusively in NHC-Au- NHC complexes lying flat on the surface. Our study highlights how well-defined structural modifications of NHCs allow to control the local binding motif on surfaces, which is important to design designated catalytic sites at interfaces.
H. Mönig, S. Amirjalayer, A. Timmer, Z. Hu, L. Liu, O. Díaz Arado, M. Cnudde, C.A. Strassert, W. Ji, M. Rohlfing and H. Fuchs
Quantitative assessment of intermolecular interactions by atomic force microscopy imaging using copper oxide tips
Nature Nanotechnology 13, 371–375 (2018)
Abstract
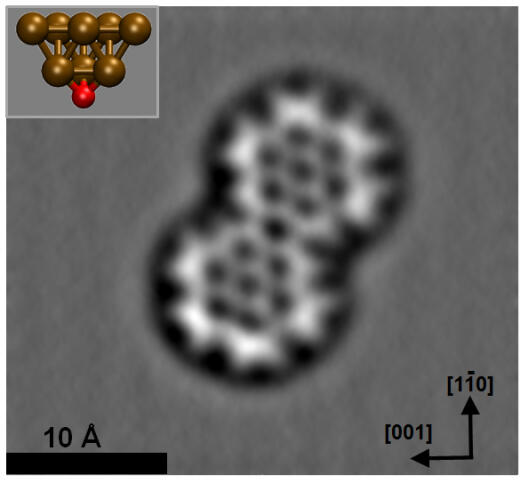
Atomic force microscopy is an impressive tool with which to directly resolve the bonding structure of organic compounds. The methodology usually involves chemical passivation of the probe-tip termination by attaching single molecules or atoms such as CO or Xe. However, these probe particles are only weakly connected to the metallic apex, which results in considerable dynamic deflection. This probe particle deflection leads to pronounced image distortions, systematic overestimation of bond lengths, and in some cases even spurious bond-like contrast features, thus inhibiting reliable data interpretation. Recently, an alternative approach to tip passivation has been used in which slightly indenting a tip into oxidized copper substrates and subsequent contrast analysis allows for the verification of an oxygen-terminated Cu tip. Here we show that, due to the covalently bound configuration of the terminal oxygen atom, this copper oxide tip (CuOx tip) has a high structural stability, allowing not only a quantitative determination of individual bond lengths and access to bond order effects, but also reliable intermolecular bond characterization. In particular, by removing the previous limitations of flexible probe particles, we are able to provide conclusive experimental evidence for an unusual intermolecular N–Au–N three-centre bond. Furthermore, we demonstrate that CuOx tips allow the characterization of the strength and configuration of individual hydrogen bonds within a molecular assembly.
D. Ji, T. Li, Y. Zou, M. Chu, K. Zhou, J. Liu, G. Tian, Z. Zhang, X. Zhang, L. Li, D. Wu, H. Dong, Q. Miao, H. Fuchs, W. Hu
Copolymer dielectrics with balanced chain-packing density and surface polarity for high-performance flexible organic electronics
Nature Communications 9, 2339 (2018)
Abstract
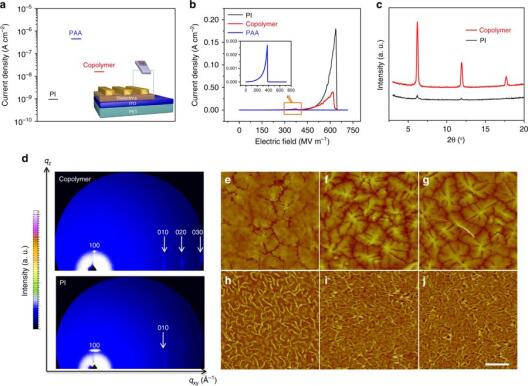
The ever-increasing demand for flexible electronics calls for the development of low-voltage and high-mobility organic thin-film transistors (OTFTs) that can be integrated into emerging display and labeling technologies. Polymer dielectrics with comprehensive and balanced dielectric properties (i.e., a good balance between their insulating characteristics and compatibility with organic semiconductors) are considered particularly important for this end. Here, we introduce a simple but highly efficient strategy to realize this target by using a new type of copolymer as dielectrics. Benefiting from both high chain packing density guaranteeing dielectric properties and surface polarity optimizing molecular packing of organic semiconductors, this rationally designed copolymer dielectric endows flexible OTFTs with high mobility (5.6 cm2 V−1 s−1), low operating voltage (3 V) and outstanding stability. Further, their applicability in integrated circuits is verified. The excellent device performance shows exciting prospects of this molecular-scale engineered copolymer for the realization of plastic high-performance integrated electronics.
J. Atwater, D. S. Mattes, B. Streit, C. von Bojničić‐Kninski, F. F. Loeffler, F. Breitling, H. Fuchs, M. Hirtz
Combinatorial Synthesis of Macromolecular Arrays by Microchannel Cantilever Spotting (µCS)
Adv. Mater. 1801632 (2018)
Abstract
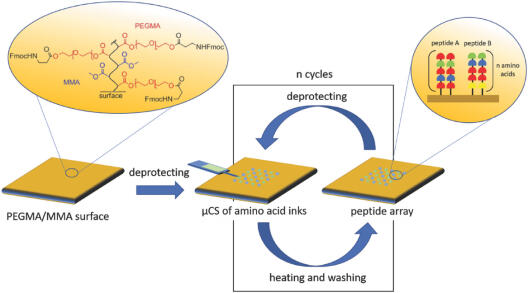
Surface‐bound microarrays of multiple oligo‐ and macromolecules (e.g., peptides, DNA) offer versatile options in biomedical applications like drug screening, DNA analysis, or medical diagnostics. Combinatorial syntheses of these molecules in situ can save significant resources in regard to processing time and material use. Furthermore, high feature densities are needed to enable high‐throughput and low sample volumes as generally regarded in combinatorial chemistry. Here, a scanning‐probe‐lithography‐based approach for the combinatorial in situ synthesis of macromolecules is presented in microarray format. Feature sizes below 40 µm allow for the creation of high‐density arrays with feature densities of 62 500 features per cm2. To demonstrate feasibility of this approach for biomedical applications, a multiplexed array of functional protein tags (HA‐ and FLAG‐tag) is synthesized, and selective binding of respective epitope recognizing antibodies is shown. This approach uses only small amounts of base chemicals for synthesis and can be further parallelized, therefore, opening up a route to flexible, highly dense, and cost‐effective microarrays.
L. Liu, H. Klaasen, A. Timmer, H.-Y. Gao , D. Barton, H. Mönig , J. Neugebauer, H. Fuchs, A. Studer
α-Diazo Ketones in On-Surface Chemistry
J. Am. Chem. Soc. 140, 6000–6005 (2018)
Abstract
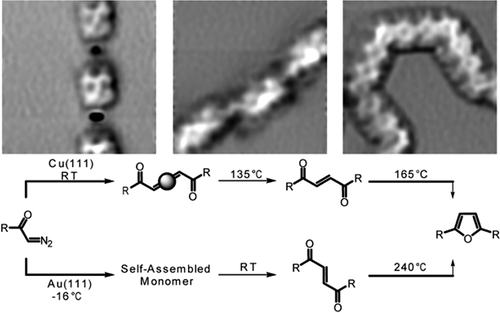
Polymerization of a biphenyl bis α-diazo ketone on Cu(111) and Au(111) surfaces to provide furandiyl bridged poly-para-phenylenes is reported. Polymerization on Cu(111) occurs via initial N2 fragmentation leading to Cu-biscarbene complexes at room temperature as polymeric organometallic structure. At 135 °C, carbene coupling affords polymeric α,β-unsaturated 1,4-diketones, while analogous alkene formation on the Au(111) surface occurs at room temperature. Further temperature increase leads to deoxygenative cyclization of the 1,4-diketone moieties to provide alternating furandiyl biphenyl copolymers on Cu(111) (165 °C) and Au(111) (240 °C) surfaces. This work shows a new approach to generate Cu-biscarbene intermediates on surfaces, opening the pathway for the controlled generation of biphenyl copolymers.
H. Lu, Y. Cao, J. Qi, A. Bakker, C.A. Strassert, X. Lin, K.-H. Ernst, S. Du, H. Fuchs, H.-J. Gao
Modification of the Potential Landscape of Molecular Rotors on Au(111) by the Presence of an STM Tip
Nano Lett., 2018, 18 (8), pp 4704–4709
Abstract
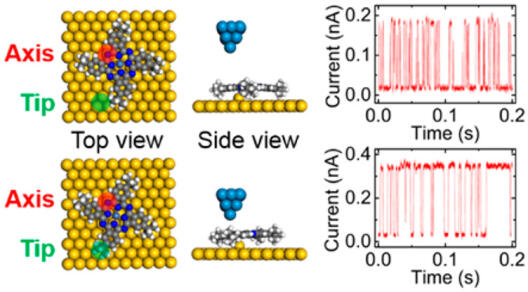
Molecular rotors on solid surfaces are fundamental components of molecular machines. No matter whether the rotation is activated by heat, electric field or light, it is determined by the intrinsic rotational potential landscape. Therefore, tuning the potential landscape is of great importance for future applications of controlled molecular rotors. Here, using scanning tunneling microscopy (STM), we demonstrate that both tip–molecule distance and sample bias can modify the rotational potential of molecular rotors. We achieve the potential energy difference variations of ∼0.3 meV/pm and ∼18 meV/V between two configurations of a molecular rotor, a tetra-tert-butyl nickel phthalocyanine molecule on Au(111) substrate. Further analysis indicates that the mechanism of modifying the rotational potential is a combination of the van der Waals interaction and the interaction between the molecular dipole and an electric field. This work provides insight into the methods used to modify the effective rotational potential energy of molecular rotors.
A.Timmer, H. Moenig, M. Uphoff, O. Díaz Arado, S. Amirjalayer, H. Fuchs
Site-specific adsorption of aromatic molecules on a metal/ metal oxide phase boundary
Nano Letters 18, 4123-4129 (2018)
Abstract
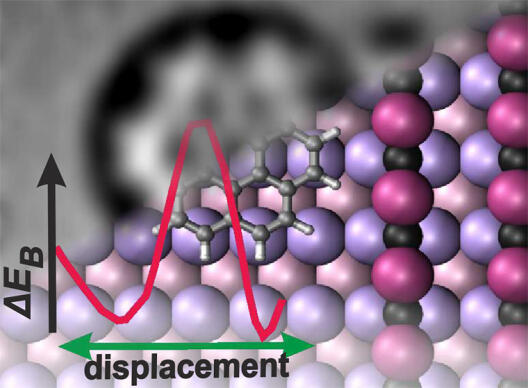
Nano-structured surfaces are ideal templates to control the self-assembly of molecular structures towards well-defined functional materials. To understand the initial adsorption process, we have investigated the arrangement and configuration of aromatic hydrocarbon molecules on nano-structured substrates composed of an alternating arrangement of Cu(110) and oxygen-reconstructed stripes. Scanning tunneling microscopy reveals a preferential adsorption of molecules at oxide phase boundaries. Non-contact atomic force microscopy experiments provide a detailed insight into the preferred adsorption site. By combining sub-molecular resolution imaging with density functional theory calculations, the interaction of the molecule with the phase boundary was elucidated, excluding a classical hydrogen bonding. Instead, a complex balance of different interactions is revealed. Our results provide an atomistic picture for the driving forces of the adsorption process. This comprehensive understanding enables developing strategies for the bottom-up growth of functional molecular systems using nano-templates.
Q. Shen, H.Y. Gao, H. Fuchs
Frontiers of on-surface synthesis: From principles to applications
Nano Today 13, 77-96 (2017)
Abstract Download full article
On-surface synthesis is the bottom-up construction of covalent bonds between molecular building blocks, which has been greatly developed during the past decade. Dozens of reactions have been successfully realized and scrutinized on various surfaces with the help of surface science techniques combined with theoretical calculations. Functional nanoarchitectures such as one-dimensional nanowires, nanoribbons and two-dimensional nanonetworks have been constructed on surfaces and explored in several potential applications. In fact, the generation of multilevel nanostructures will play a key role in future soft nanoscience and technologies due to their emergent properties ranging far beyond those of the individual molecules building them up. In this review, we discuss selected examples of important processes in on-surface synthesis developed in recent years and summarize them under the following aspects:
- on-surface reactions in a category of different carbon types;
- techniques applied in on-surface synthesis;
- on-surface synthesized functional nanostructures; and
- potential applications of on-surface synthesized nano-materials.
The review concludes with a perspective of the future development of on-surface synthesis.
H.-Y. Gao, P. A. Held, S. Amirjalayer, L. Liu, A. Timmer, B. Schirmer, O. Díaz Arado, H. Mönig, C. Mück-Lichtenfeld, J. Neugebauer, A. Studer, H. Fuchs
Intermolecular On-Surface σ‑Bond Metathesis
J. Am. Chem. Soc. 139 (20), pp 7012–7019 (2017)
Abstract
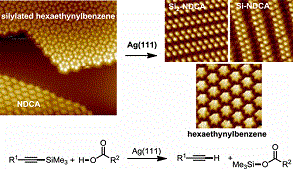
Silylation and desilylation are important functional group manipulations in solution-phase organic chemistry that are heavily used to protect/deprotect different functionalities. Herein, we disclose the first examples of the σ-bond metathesis of silylated alkynes with aromatic carboxylic acids on the Ag(111) and Au(111) surfaces to give the corresponding terminal alkynes and silyl esters, which is supported by density functional theory calculations and further confirmed by X-ray photoelectron spectroscopy analysis. Such a protecting group strategy applied to on-surface chemistry allows self-assembly structures to be generated from molecules that are inherently unstable in solution and in the solid state. This is shown by the successful formation of self-assembled hexaethynylbenzene at Ag(111). Furthermore, it is also shown that on the Au(111) surface this σ-bond metathesis can be combined with Glaser coupling to fabricate covalent polymers via a cascade process.
H. Kong, S. Yang, H.-Y. Gao, A. Timmer, J. P. Hill, O. Díaz Arado, H. Mönig, X. Huang, Q. Tang, Q. Ji, W. Liu, H. Fuchs
Substrate Mediated C-C and C-H Coupling after Dehalogenation
J. Am. Chem. Soc. 139 (10), pp 3669–3675 (2017)
Abstract
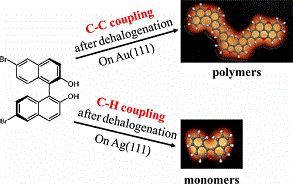
Intermolecular C–C coupling after cleavage of C–X (mostly, X = Br or I) bonds has been extensively studied for facilitating the synthesis of polymeric nanostructures. However, the accidental appearance of C–H coupling at the terminal carbon atoms would limit the successive extension of covalent polymers. To our knowledge, the selective C–H coupling after dehalogenation has not so far been reported, which may illuminate another interesting field of chemical synthesis on surfaces besides in situ fabrication of polymers, i.e., synthesis of novel organic molecules. By combining STM imaging, XPS analysis, and DFT calculations, we have achieved predominant C–C coupling on Au(111) and more interestingly selective C–H coupling on Ag(111), which in turn leads to selective synthesis of polymeric chains or new organic molecules.
D. Ji, X. Xu, L. Jiang, S. Amirjalayer, L. Jiang, Y. Zhen, Y. Zou, Y. Yao, H. Dong, J. Yu, H. Fuchs, W. Hu
Surface polarity and self-structured nanogrooves collaborative oriented molecular packing for high crystallinity towards efficient charge transport
J. Am. Chem. Soc. 139 (7), pp 2734–2740 (2017)
Abstract
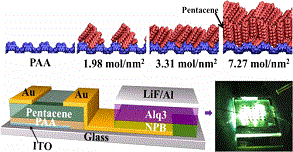
Efficient charge transport in organic semiconductors is essential for construction of high performance optoelectronic devices. Herein, for the first time, we demonstrate that poly(amic acid) (PAA), a facilely deposited and annealing-free dielectric layer, can tailor the growth of organic semiconductor films with large area and high crystallinity toward efficient charge transport and high mobility in their thin film transistors. Pentacene is used as a model system to demonstrate the concept with mobility up to 30.6 cm2 V–1 s–1, comparable to its high quality single crystal devices. The structure of PAA has corrugations with OH groups pointing out of the surface, and the presence of an amide bond further allows adjacent polymer strands to interact via hydrogen bonding, leading to a self-rippled surface perpendicular to the corrugation. On the other hand, the strong polar groups (−COOH/–CONH) of PAA could provide repulsive forces between PAA and pentacene, which results in the vertical orientation of pentacene on the dielectric surface. Indeed, in comparison with its imidized counterpart polyimide (PI), PAA dielectric significantly enhances the film crystallinity, drastically increases the domain size, and decreases the interface trap density, giving rise to superior device performance with high mobility. This concept can be extended to more organic semiconducting systems, e.g., 2,6-diphenylanthracene (DPA), tetracene, copper phthalocyanine (CuPc), and copper hexadecafluorophthalocyanine (F16CuPc), demonstrating the general applicability. The results show the importance of combining surface nanogrooves with the strong polarity in orienting the molecular arrangement for high crystallinity toward efficient charge transport in organic semiconductors.
S. Amirjalayer, A. Martinez-Cuezva, J. Berna, S. Woutersen, W. Buma
Photoinduced Pedalo-Type Motion in an Azodicarboxamide-Based Molecular Switch
Angew. Chem. Int. Ed. 57, 1792-1796 (2018)
Abstract
Angew. Chem. 130, 1810-1814 (2018)
Abstract

Well‐defined structural changes of molecular units that can be triggered by light are crucial for the development of photoactive functional materials. Herein, we report on a novel switch that has azodicarboxamide as its photo‐triggerable element. Time‐resolved UV‐pump/IR probe spectroscopy in combination with quantum‐chemical calculations shows that the azodicarboxamide functionality, in contrast to other azo‐based chromophores, does not undergo trans–cis photoisomerization. Instead, a photoinduced pedalo‐type motion occurs, which because of its volume‐conserving properties enables the design of functional molecular systems with controllable motion in a confined space.

