Publications
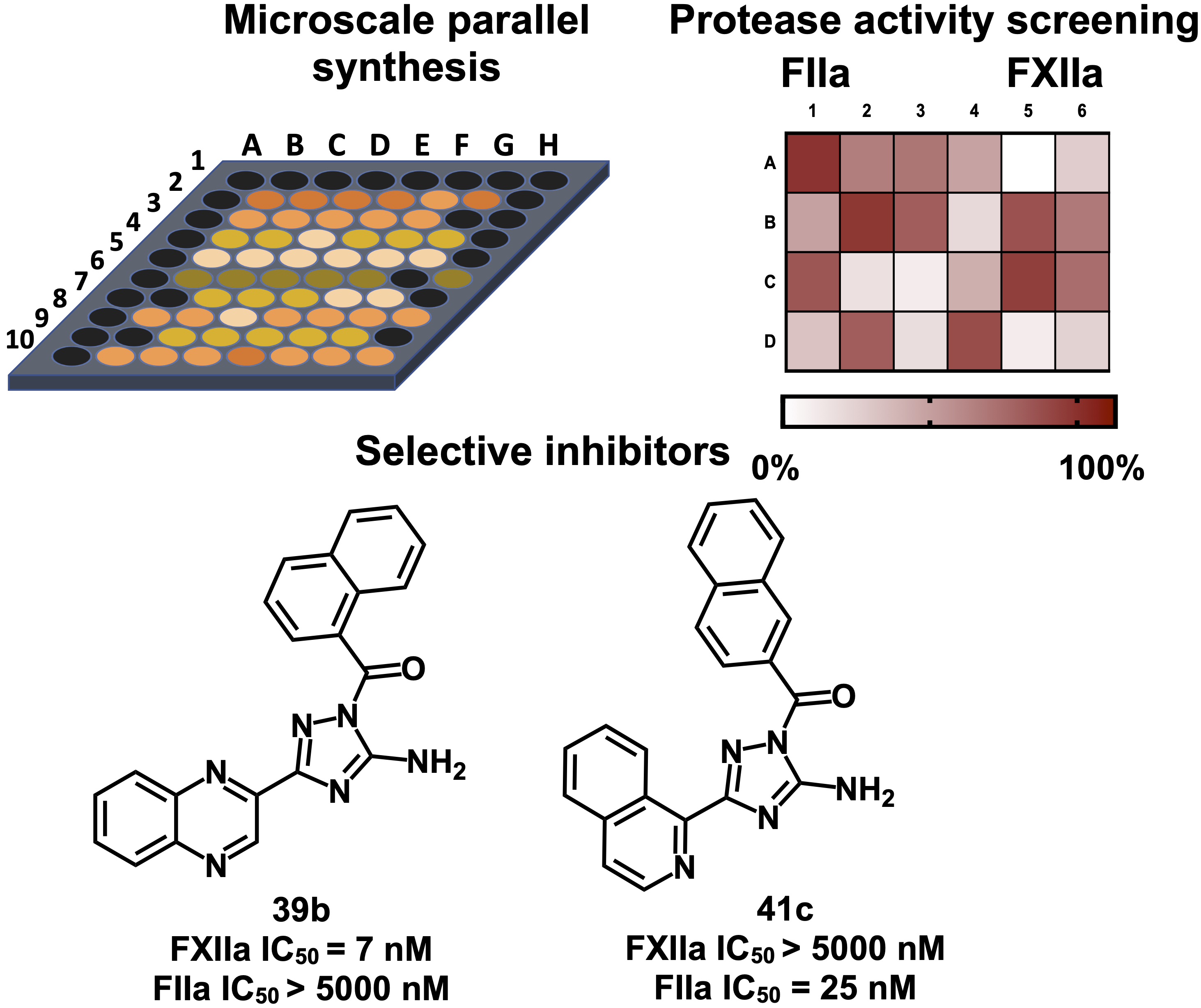
1. Platte, S., Korff, M., Imberg, L., Balicioglu, I., Erbacher, C., Will, J.M., Daniliuc, C.G. and Karst, U., Kalinin, D.V.* Microscale Parallel Synthesis of Acylated Aminotriazoles Enabling the Development of Factor XIIa and Thrombin Inhibitors. ChemMedChem 2021. https://doi.org/10.1002/cmdc.202100431
2. Kalinin, D. V.* Factor XII(a) inhibitors: a review of the patent literature. Expert Opin Ther Pat 2021 (just-accepted). https://doi.org/10.1080/13543776.2021.1945580
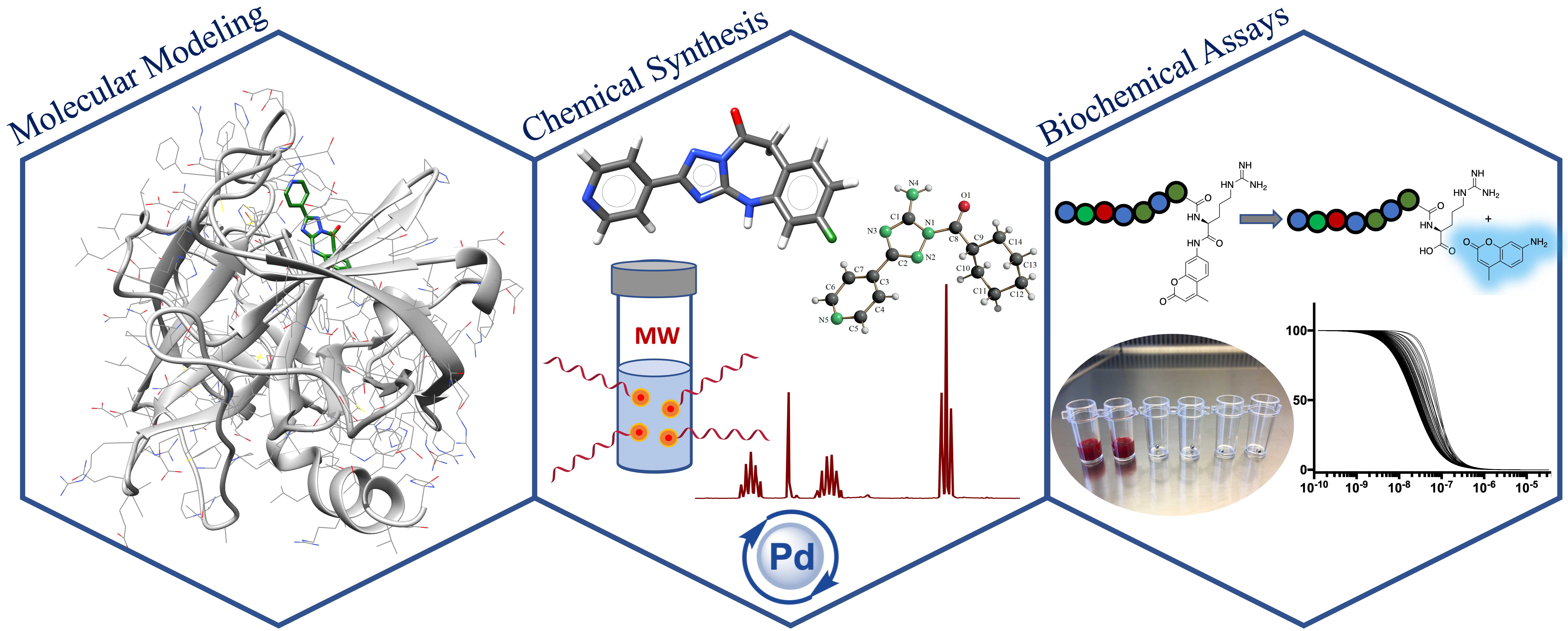
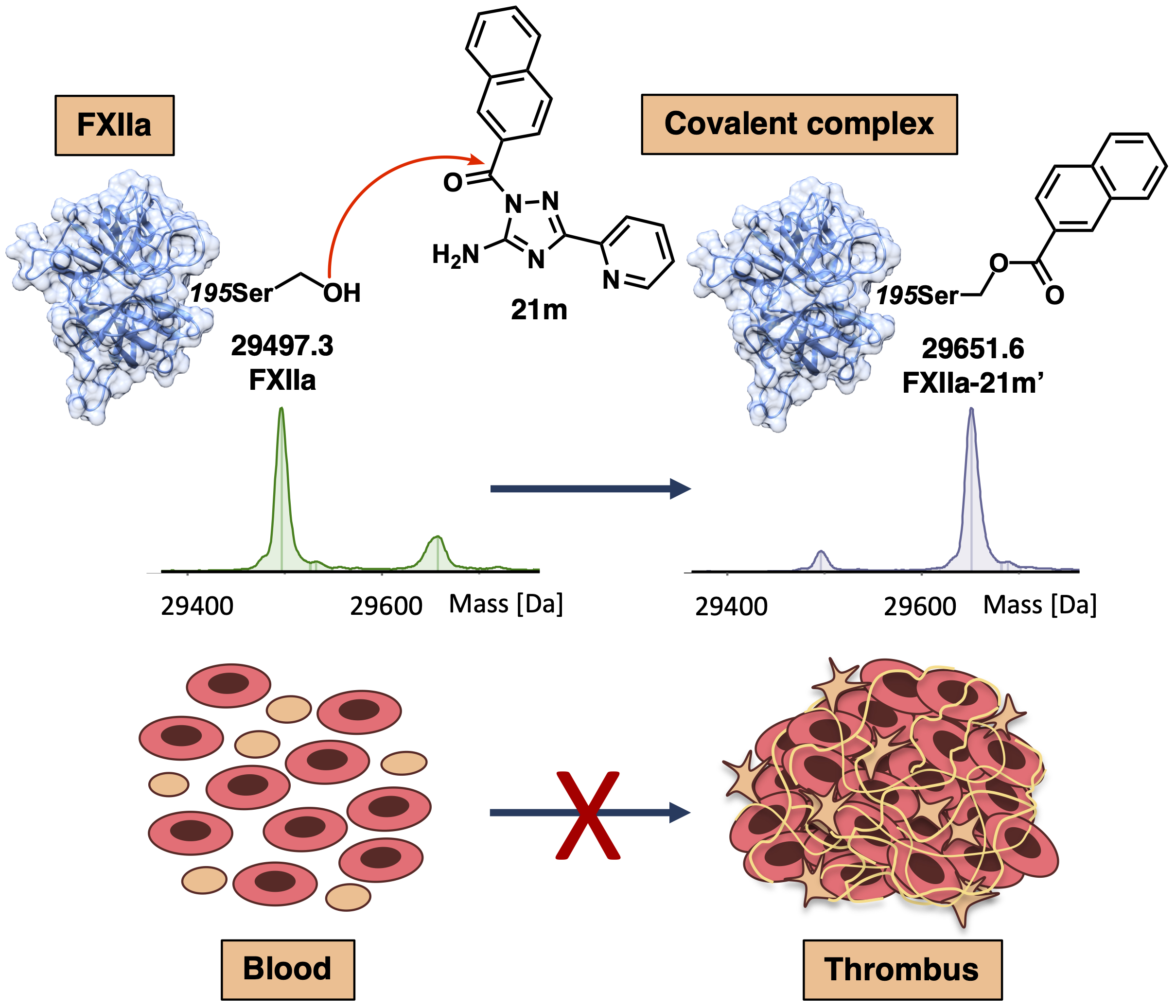
3. Korff, M.; Imberg, L.; Will, J. M.; Buckreiss, N.; Kalinina, S. A.; Wenzel, B. M.; Kastner, G. A.; Daniliuc, C. G.; Barth, M.; Ovsepyan, R. A.; Butov, K. R.; Humpf, H. U.; Lehr, M.; Panteleev, M. A.; Poso, A.; Karst, U.; Steinmetzer, T.; Bendas, G.; Kalinin, D. V.* Acylated 1H-1,2,4-Triazol-5-amines Targeting Human Coagulation Factor XIIa and Thrombin: Conventional and Microscale Synthesis, Anticoagulant Properties, and Mechanism of Action. J Med Chem 2020, 63, 13159-13186. https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01635
4. Kalinin, D. V.; Jana, S. K.; Pfafenrot, M.; Chakrabarti, A.; Melesina, J.; Shaik, T. B.; Lancelot, J.; Pierce, R. J.; Sippl, W.; Romier, C.; Jung, M.; Holl, R. Structure-Based Design, Synthesis, and Biological Evaluation of Triazole-Based smHDAC8 Inhibitors. ChemMedChem 2020, 15, 571-584. https://doi.org/10.1002/cmdc.201900583
5. Tangherlini, G.; Kalinin, D. V.; Schepmann, D.; Che, T.; Mykicki, N.; Stander, S.; Loser, K.; Wunsch, B. Development of Novel Quinoxaline-Based kappa-Opioid Receptor Agonists for the Treatment of Neuroinflammation. J Med Chem 2019, 62, 893-907. https://doi.org/10.1021/acs.jmedchem.8b01609
6. Kalinin, D. V.; Agoglitta, O.; Van de Vyver, H.; Melesina, J.; Wagner, S.; Riemann, B.; Schafers, M.; Sippl, W.; Loffler, B.; Holl, R. Proline-based hydroxamates targeting the zinc-dependent deacetylase LpxC: Synthesis, antibacterial properties, and docking studies. Bioorg Med Chem 2019, 27, 1997-2018. https://doi.org/10.1016/j.bmc.2019.03.056
7. Thum, S.; Schepmann, D.; Kalinin, D. V.; Ametamey, S. M.; Wunsch, B. Replacement of the Benzylpiperidine Moiety with Fluorinated Phenylalkyl Side Chains for the Development of GluN2B Receptor Ligands. ChemMedChem 2018, 13, 2522-2529. https://doi.org/10.1002/cmdc.201800566
8. Marek, M.; Shaik, T. B.; Heimburg, T.; Chakrabarti, A.; Lancelot, J.; Ramos-Morales, E.; Da Veiga, C.; Kalinin, D.; Melesina, J.; Robaa, D.; Schmidtkunz, K.; Suzuki, T.; Holl, R.; Ennifar, E.; Pierce, R. J.; Jung, M.; Sippl, W.; Romier, C. Characterization of Histone Deacetylase 8 (HDAC8) Selective Inhibition Reveals Specific Active Site Structural and Functional Determinants. J Med Chem 2018, 61, 10000-10016. https://doi.org/10.1021/acs.jmedchem.8b01087
9. Kalinina, S. A.; Kalinin, D. V.; Hovelmann, Y.; Daniliuc, C. G.; Muck-Lichtenfeld, C.; Cramer, B.; Humpf, H. U. Auranthine, a Benzodiazepinone from Penicillium aurantiogriseum: Refined Structure, Absolute Configuration, and Cytotoxicity. J Nat Prod 2018, 81, 2177-2186. https://doi.org/10.1021/acs.jnatprod.8b00187
10. Kalinin, D. V.; Holl, R. LpxC inhibitors: a patent review (2010-2016). Expert Opin Ther Pat 2017, 27, 1227-1250. https://doi.org/10.1080/13543776.2017.1360282
11. Kalinin, D. V.; Wagner, S.; Riemann, B.; Hermann, S.; Schmidt, F.; Becker-Pauly, C.; Rose-John, S.; Schafers, M.; Holl, R. Novel Potent Proline-Based Metalloproteinase Inhibitors: Design, (Radio)Synthesis, and First in Vivo Evaluation as Radiotracers for Positron Emission Tomography. J Med Chem 2016, 59, 9541-9559. https://doi.org/10.1021/acs.jmedchem.6b01291
12. Kalinin, D. V.; Holl, R. Insights into the Zinc-Dependent Deacetylase LpxC: Biochemical Properties and Inhibitor Design. Curr Top Med Chem 2016, 16, 2379-430. https://doi.org/10.2174/1568026616666160413135835
13. Kalinina, S. A.; Elkina, O. V.; Kalinin, D. V.; Syropyatov, B. Y.; Dolzhenko, A. V. Diuretic activity and toxicity of some Verbascum nigrum extracts and fractions. Pharm Biol 2014, 52, 191-8. https://doi.org/10.3109/13880209.2013.822001
14. Kalinina, S. A.; Kalinin, D. V.; Dolzhenko, A. V. A one-pot, three-component, microwave-promoted synthesis of 2-amino-substituted 7-amino-1,2,4-triazolo[1,5-a][1,3,5]triazines. Tetrahedron Lett 2013, 54, 5537-5540. https://doi.org/10.1016/j.tetlet.2013.07.158
15. Kalinin, D. V.; Pantsurkin, V. I.; Syropyatov, B. Y.; Kalinina, S. A.; Rudakova, I. P.; Vakhrin, M. I.; Dolzhenko, A. V. Synthesis, local anaesthetic and antiarrhythmic activities of N-alkyl derivatives of proline anilides. Eur J Med Chem 2013, 63, 144-50. https://doi.org/10.1016/j.ejmech.2013.02.003
16. Kalinin, D. V.; Kalinina, S. A.; Dolzhenko, A. V. A New Synthesis of Amino Substituted Azolo[1,3,5]Triazines Via Reaction of N-1,N-1-Dimethyl-N-2-Azolylformamidines with Cyanamide. Heterocycles 2013, 87, 147-154. http://doi.org/10.3987/COM-12-12601
17. Dolzhenko, A. V.; Kalinina, S. A.; Kalinin, D. V. A novel multicomponent microwave-assisted synthesis of 5-aza-adenines. Rsc Adv 2013, 3, 15850-15855. https://doi.org/10.1039/C3RA41932K
18. Kalinin, D. V.; Kalinina, S. A.; Dolzhenko, A. V. Synthesis of Novel Trichloromethyl Substituted Azolo[1,3,5]Triazines. Heterocycles 2012, 85, 2515-2522. http://doi.org/10.3987/COM-12-12542