Molecular function of tumor suppressor complexes
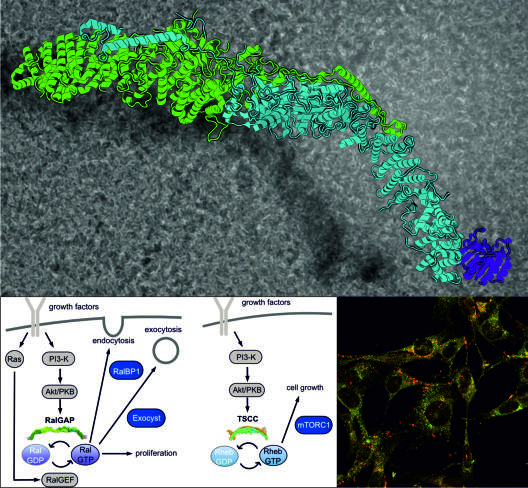
The small GTPases Ral and Rheb are molecular switches that regulate cellular processes like signal transduction and vesicular transport. Their dysregulation is involved in a variety of diseases, including oncogenic signaling and neurological disorders. The RalGAP and TSC complexes are GTPase activating protein (GAPs) complexes that counteract Ral and Rheb activity, respectively, and therefore serve as important tumor suppressors. One major focus of our research is the structural and functional investigation of the TSC and RalGAP complexes to understand their mechanistic roles in physiology and pathology.
Rasche R. et. al (2025)
Structure and mechanism of the RalGAP tumor suppressor complex.
Nat Commun doi.org/10.1038/s41467-025-61743-9.
Fitzian et. al (2021)
TSC1 binding to lysosomal PIPs is required for TSC complex translocation and mTORC1 regulation.
Mol Cell doi: 10.1016/j.molcel.2021.04.019
Hansmann et al (2020)
Structure of the TSC2 GAP Domain: Mechanistic Insight into Catalysis and Pathogenic Mutations.
Structure doi: 10.1016/j.str.2020.05.008