Coordination of membrane transport processses and fusion
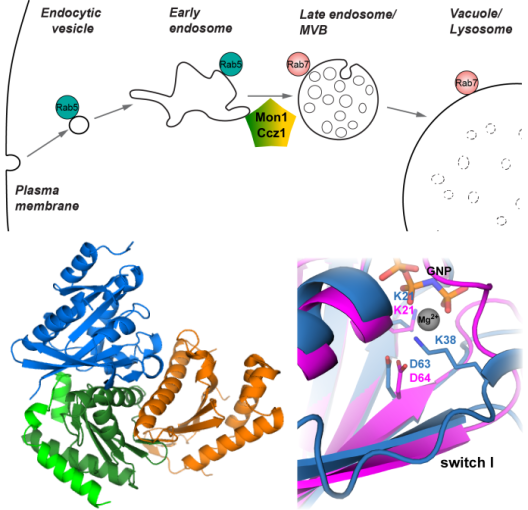
Extracellular substances and cell surface proteins are internalized by endocytic vesicles, which fuse to form early endosome. At these organelles cargo is sorted and recycled. Alternatively, the content is delivered to the lysosome for degradation. This process requires the maturation of Rab5-positive early endosomes into a late endosome through the conversion into a Rab7-positive membrane. The molecular mechanism of this process is poorly understood, but the Mon1-Ccz1 (MC1) complex has been identified as a key player: MC1 is recruited by Rab5 and acts as GEF (activator) for Rab7 and thus initiates organelle conversion. We study the structure and function of MC1 to gain better insight into the mechanism and regulation of endosomal maturation. In addition, we are interested in the related CPLANE complex that controls ciliogenesis and is associated with developmental disease.
Wilmes S, et al. (2025)
Mechanistic adaptation of the metazoan RabGEFs Mon1-Ccz1 and Fuzzy-Inturned.
Sci Adv doi.org/10.1126/sciadv.adx2893
Herrmann E., et al. (2023)
Structure of the metazoan Rab7 GEF complex Mon1-Ccz1-Bulli.
Proc Natl Acad Sci USA doi.org/10.1073/pnas.2301908120
Kiontke et al. (2017)
Architecture and mechanism of the late endosomal Rab7-like Ypt7 guanine nucleotide exchange factor complex Mon1-Ccz1.
Nat Commun doi: 10.1038/ncomms14034