Ethylene Carbonate Free Electrolyte Extends Lifespan of High-Voltage Batteries
Until now, the solvent ethylene carbonate (EC) was considered a fundamental component of the electrolyte in lithium-ion batteries. The research results by a team led by Dr Tobias Placke from MEET Battery Research Center and Dr Johannes Kasnatscheew from Helmholtz Institute Münster (HI MS) not only proves that the electrolyte can be used without EC. The researchers also show that a longer battery cell life can be achieved with increased voltages and higher energy density when EC is excluded.
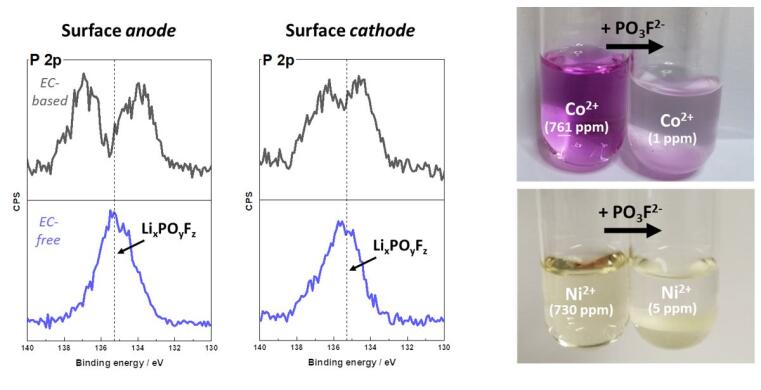
"The fluorophosphate-based species generated during battery application causes the mechanism. It ensures that the metals cobalt, nickel and manganese dissolved from the cathode are intercepted before they can damage the anode," explains Kasnatscheew. In the past, researchers have faced the challenge of the cathode decomposing at elevated voltages and metals dissolving in the electrolyte. These migrate to the anode, settle there and cause a drop in service life as well as a safety risk, as short circuits can occur. This cascade of faults can be prevented by the formation of fluorophosphates, as the collaborative work between MEET and HI MS shows. The metals are intercepted before they can even reach the anode.
New Perspectives for the Formulation and Design of Electrolytes
The improvement in lifespan at increased charge voltages and energy densities was found in a highly simplified electrolyte system with the two components lithium salt and linear carbonate, without the addition of auxiliary substances such as additives. Sven Klein from MEET is optimistic: “The findings on the possibility of modification open up new perspectives for the formulation and design of electrolytes for battery cells. They enable systematic research and development in the field of high-voltage applications, for example for electromobility and stationary energy storage.”
Omitting EC creates new freedom for electrolyte development as the electrolyte does not need to be adapted to the added EC. For example, new material combinations for lower ambient temperatures can be considered.
Detailed Results published
Sven Klein, Stefan van Wickeren, Peer Bärmann, Bastian Heidrich, Dr Markus Börner, Dr Tobias Placke (MEET), Dr Stephan Röser, Dr Kristina Borzutzki, Dr Johannes Kasnatscheew (HI MS) and Prof. Dr Martin Winter (HI MS and MEET) published their results as a cover article in the journal "Advanced Energy Materials". The article is open access.

