Strategy for Extended Life of High Energy Batteries
In their new study, researchers from MEET Battery Research Center of the University of Münster and Helmholtz Institute Münster (HI MS; IEK-12) of Forschungszentrum Jülich show how the cyclic lifetime of lithium-ion batteries (LIB) can be optimised during high voltage operation. The concentration of lithium fluorophosphates (LixPOyFz, LPF) plays a central role here.
Mitigating Failure Processes
Increasing the end-of-charge voltage leads to an increase in the energy density of LIBs, but also to a decrease in the cyclic lifetime, so that a compromise is inevitable. The dissolution of the metals nickel, cobalt and manganese from the cathode and their deposition on the anode ("electrode crosstalk") is the predominant failure mechanism.
LPFs fulfil the task of mitigating this process in the battery cell. They scavenge the metals before they can reach and damage the anode. It has now been shown what influence the concentration of LPF has on the service life of the LIB.
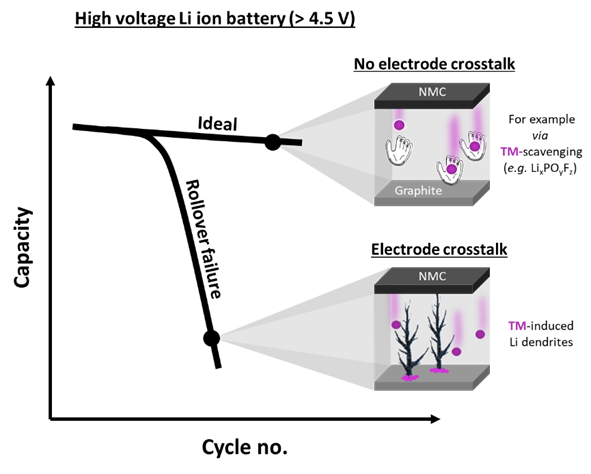
Intrinsic Formation of LPF
"One challenge in our research is a possible intrinsic formation of LPFs at certain conditions and can have a decisive influence on the overall concentration," explains Dr Johannes Kasnatscheew from HI MS. These hidden deviations in concentration depend on other materials used.
Adding LPF to electrolyte systems above the optimal concentration can cause overdosing and even harm the performance of the LIB. Ethylene carbonate (EC)-free electrolytes, for example, pose a particular challenge because they produce a significant proportion of LPF intrinsically. In contrast, the addition of the well-known additive vinylene carbonate even leads to a reduction of the LPF concentration in EC-free systems, as it prevents the intrinsic formation of LPF.
Sven Klein from MEET Battery Research Center classifies the results: "These and similar effects must be taken into account when optimising electrolyte formulations for LIB in high-voltage applications. The potential of LIB can thus be further exploited."
Study Available in ChemElectroChem
The researchers Lukas Stolz, Dr Kristina Borzutzki, Dr Johannes Kasnatscheew, HI MS, Sven Klein, Lukas Haneke, Patrik Harte, Stefan van Wickeren, Dr Sascha Nowak, MEET Battery Research Centre, and Prof. Dr Martin Winter, HI MS and MEET Battery Research Center, published the detailed results of their study as an open access article in the journal ChemElectroChem.

