New Avenue for Aqueous Processing of Cathode Materials
A more sustainable production of battery cells and the electrodes used is increasingly becoming the focus of research. One approach is to use aqueous routes in cathode production instead of expensive, state-of-the-art toxic organic solvents such as N-methyl-2-pyrrolidone (NMP) and fluorinated binders. However, this more environmentally friendly method has so far resulted in poorer battery performance. Scientists at MEET Battery Research Center of the University of Münster and Helmholtz Institute Münster (HI MS; IEK-12) of Forschungszentrum Jülich have developed a new method for processing nickel-rich lithium-nickel-cobalt-manganese (NCM) electrodes that overcomes previous hurdles.
Binder System Prevents Performance Loss
When water is used as a solvent for processing cathode materials, a lithium ion (Li+) / hydrogen ion (H+) exchange occurs as soon as NCM particles are in contact with water. Lithium leaching causes a rapid increase of the pH value of the aqueous electrode paste ultimately leading to the corrosion of the aluminium foil current collector. As a result, the electrochemical performance of the cell drops dramatically.
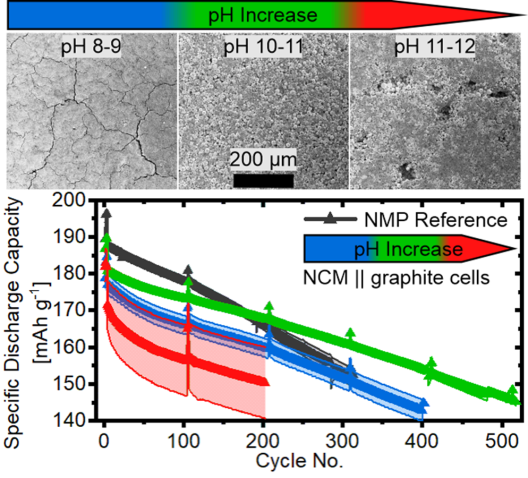
The interdisciplinary team of MEET and HI MS researchers succeeded in developing a new route for aqueous processing of Ni-rich NCM electrodes. A binder system consisting of lithium hydroxide (LiOH) and polyacrylic acid (PAA) plays a central role. By using different molar ratios of LiOH/PAA, a stabilization of the pH value of the electrode pastes could be achieved. This prevents corrosion of the aluminium current collector and achieves a high initial capacity with good cycling stability. Friederike Reissig, researcher at Helmholtz Institute Münster, explains: “Electrochemical studies in graphite-based lithium-ion battery full cells of the aqueous processed cathodes showed long-term cycling stability comparable to classically processed electrodes. Thus, cathode fabrication can be more environmentally friendly and possible without battery performance degradation.”
Complete Study Available in ChemSusChem
The the detailed results of their study have been published by researchers Friederike Reissig, Helmholtz Institute Münster, Sebastian Puls, Dr Tobias Placke, Dr Richard Schmuch and Dr Aurora Gomez-Martin, MEET Battery Research Center, and Prof Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster, in the journal “ChemSusChem”.

