First Analytical Study of Oxidative Degradation Process of Film-forming Additives
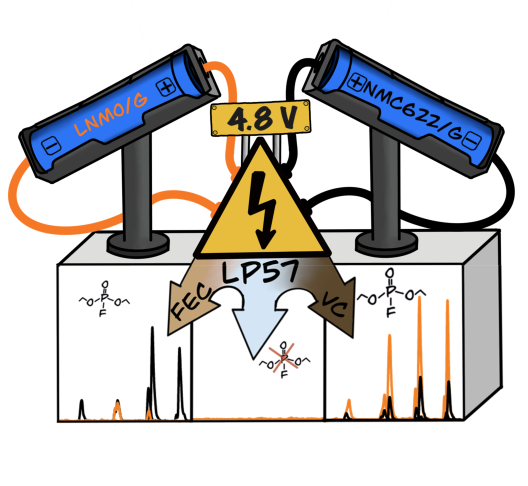
Increasing the cell voltage is considered a simple approach to boost the specific energy in lithium-ion batteries, since no additional production steps are required. However, the resulting effects on the electrolyte and electrode materials are not sufficiently researched to transfer the concept into practice. A team from MEET Battery Research Center at the University of Münster has therefore analyzed the stability of the electrolyte and the film-forming additives fluoroethylene carbonate (FEC) and vinylene carbonate (VC) under high voltage for the first time. In addition, the scientists investigated how the cathode materials influence the degradation process of the electrolyte.
Film-forming Additive and Cathode Material Selection Crucial
"We cannot develop approaches that increase the stability and safety of high-voltage LIBs until we have a precise understanding of the reactions taking place within the cell," explains MEET scientist Maximilian Kubot. The film-forming additive VC is not stable under high voltage. Above a cell voltage of 4.7 volts, the liquid degrades oxidatively. Through this process and further reactions with the electrolyte, highly toxic organofluorophosphates (OFP) are formed.
Due to its higher electrochemical stability, the additive FEC does not oxidize initially. "The decisive factor at this point is the selection of the cathode material," says Kubot. Comparing nickel-rich nickel cobalt manganese (NMC622) with high voltage lithium nickel manganese oxide (LNMO), cells with NMC622 cathode have a significantly higher OFP formation than cells with LNMO cathode. The reason is the reactive cathode surface of NMC622, which reacts with the electrolyte. An irreversible phase transformation of the crystal structures on the surface occurs, which releases a reactive oxygen species. This results in chemical oxidation of the FEC. Kubot concludes: "In high-voltage LIBs, the formation of highly toxic OFP is a problem that we can solve by the appropriate selection of electrode materials and film-forming additives."
Study Online Open Access
Detailed results of their study have been published by Maximilian Kubot, Bastian von Holtum, Dr Sascha Nowak and Dr Simon Wiemers-Meyer, MEET Battery Research Center, and Prof Dr Martin Winter, MEET Battery Research Center as well as Helmholtz Institute Münster of Forschungszentrum Jülich, in the scientific journal "Advanced Energy Materials".

