Compatibility of Lithium Squarate as Electrolyte Additive for Prelithiation Investigated
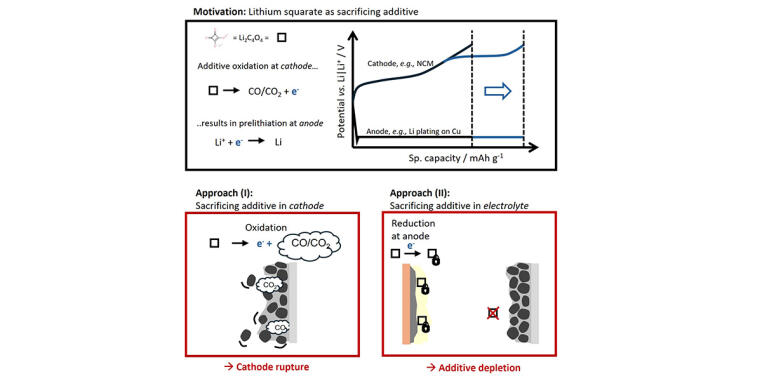
Prelithiation via sacrificing additives is considered particularly attractive in battery research because it is easily scalable and cost-effective. In this process, the sacrificing additive oxidizes, thereby providing additional electrons and thus active lithium. The resulting extra capacity can counteract the rapid capacity loss of some battery systems. A prominent sacrificing additive for lithium ion batteries is lithium squarates, which is typically incorporated via the cathode. However, gas is formed during their oxidation, which damages the cathode composite. To avoid this, a team from MEET Battery Research Center at the University of Münster investigated whether lithium squarates can also be used as electrolyte additives.
Oxidation at the Cathode Does Not Occur
The scientists' research proved that the material is not suitable as an electrolyte additive. The reason is that lithium squarate reductively depletes at the anode in course of solid electrolyte interphase (SEI) formation, the protective layer between the liquid electrolyte and the solid electrode. The aimed oxidation at the cathode is then not possible. “This knowledge helps in selecting more suitable sacrificing additives,” explains Ibrahim Lawan Abdullahi, PhD student at MEET Battery Research Center at the University of Münster and the International Graduate School BACCARA. In addition to a suitable oxidation potential of the cathode, they need to be less reductive at the anode as well so that the additive is not depleted.
Dr Johannes Kasnatscheew, Head of the Research Division Materials at MEET Battery Research Center, adds: “Prelithiation can be a key enabler, especially for next-generation batteries such as zero-excess lithium metal batteries or cells with silicon anodes, as it compensates for high capacity losses.” However, the process is sensitive and increases costs due to additional processing steps. “This is precisely why prelithiation using sacrificing additives is so interesting. It is simple and economically attractive. However, research and development are still needed to understand and circumvent the technical hurdles,” says Kasnatscheew.
Entire Study Available
The detailed results have been published by the authors Ibrahim Lawan Abdullahi, Dr Anindityo Arifiadi, Alexandros Tsoufios, Nick Fehlings, Silvan Stuckenberg, Dr Lukas Stolz, Dr Dominik Voigt and Dr Johannes Kasnatscheew, MEET Battery Research Center, as well as Prof. Dr Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster of Forschungszentrum Jülich, in the journal “Advanced Science”.

