Decisive factor identified, that influences the efficiency of lithium metal batteries
In addition to high purchase costs and the still underdeveloped charging infrastructure, the limited range is one of the biggest obstacles to switching to electric vehicles. Industry and science are therefore working intensively on developing batteries with a higher energy density and making them suitable for series production. One of the most promising alternatives to the currently common lithium ion battery (LIB) is the lithium metal battery (LMB). In contrast to the LIB, in which the presently used graphite anodes limit the energy density of the battery, the LMB promises significantly higher performance due to the use of high capacity lithium metal-based anodes.
However, despite intensive research, the LMB still carries major safety risks, which limit its broad commercial application together with its low efficiency. Inside the cell, uncontrolled lithium metal deposition occurs. This results in the formation of so-called dendrites, which cause the cell to lose its capacity during operation. In addition, the risk of short circuits increases, in the worst case the cell catches fire. Now a team led by MEET scientists Aleksei Kolesnikov and Dr. Marian Cristian Stan has identified another factor that decisively influences the efficiency of LMBs: galvanic corrosion.
The influence of galvanic corrosion on the efficiency of lithium metal batteries
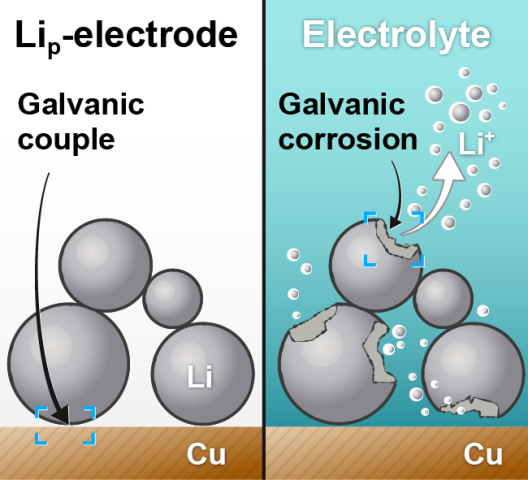
At the anode of the investigated LMB, lithium metal in powder form was applied to a copper foil. When these two different metals meet a corrosive liquid, the more active material - in this case lithium - is degraded. The process is known as galvanic corrosion. The battery cell contains exactly such a liquid, with the electrolyte essential for its proper functioning.
“Using the example of electrodes based on lithium powder, we have demonstrated that galvanic corrosion occurs in the lithium metal battery,” explains MEET scientist Aleksei Kolesnikov. “For this purpose, we aged the electrodes in a liquid electrolyte for one week. During this process, the lithium dissolved very quickly in several cases.” The cell thus loses capacity, which in turn reduces the maximum number of charge/discharge cycles of the LMB.
However, Kolesnikov does not see the results as the termination of the lithium metal battery: “Instead, galvanic corrosion must be considered in further research - also in the current development of so-called anode-free batteries. In these batteries, the lithium is not in the anode, but passes from the cathode to the anode during charging. There it meets the copper current collector. Together with the electrolyte, galvanic corrosion is therefore also possible in such batteries.”
Entire study published in scientific journal
The results of the research by Aleksei Kolesnikov and the co-authors Martin Kolek, Jan Frederik Dohmann, Fabian Horsthemke, Dr. Markus Börner, Dr. Peter Bieker, Prof. Dr. Martin Winter and Dr. Marian Cristian Stan have now been published as a cover story in the scientific journal "Advanced Energy Materials". Read the entire study here.

