Why Single-Crystal Cathode Materials Increase the Lifetime in High-Voltage Batteries
New insights into chemical ageing processes, so-called degradation mechanisms in high-voltage lithium-ion batteries have been gained by scientists from MEET Battery Research Center at the University of Münster and from Helmholtz Institute Münster of Forschungszentrum Jülich. They discovered why the use of single-crystal cathode materials in lithium-ion batteries can lead to enhanced cycle life.
The Objective: More Energy Density for an Increased Driving Range
Lithium-ion batteries are considered to pave the way for electromobility. For their use as high-energy batteries, layered oxides based on lithium nickel cobalt manganese oxide (NCM) are currently used as cathode materials. What is needed in the future are lithium-ion batteries with a higher energy density, which can be achieved, e.g., by increasing the cell voltage.
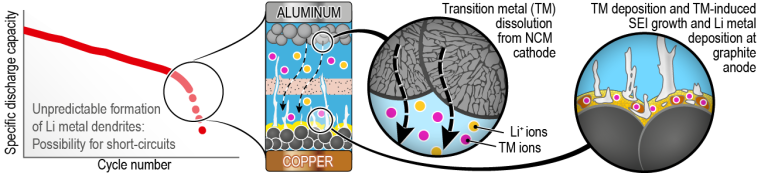
During battery operation, however, degradation mechanisms occur – in particular at high cell voltages – that result in a significant drop in performance. At present, electric vehicles can therefore not yet satisfactory keep up with combustion engines in terms of driving range. For overcoming this challenge, a comprehensive understanding of the degradation mechanisms that take place at high cell voltage (4.5 V) is essential. While previous research has provided initial insights, the complex processes have not yet been comprehensively deciphered.
Transition Metal-Induced Formation of Lithium Metal Dendrites Responsible for Performance Degradation
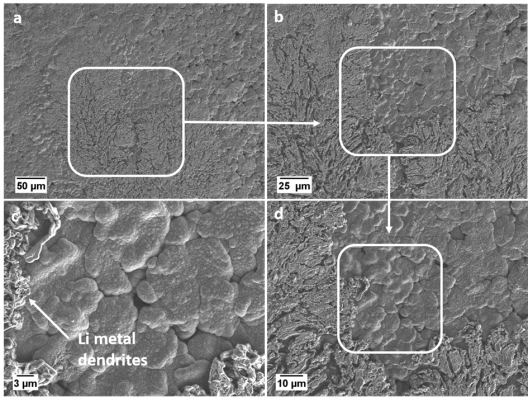
The research team led by Sven Klein, Dr. Tobias Placke and Prof. Dr. Martin Winter from MEET Battery Research Center and Helmholtz Institute Münster has now deciphered that the loss of capacity after about 50 cycles is due to a strong growth of the anode boundary layer, the so-called ‘solid electrolyte interphase’ (SEI). Deposited transition metals as cobalt, nickel and manganese that migrate from the cathode to the anode during ongoing battery operation form thick layers on the graphite anode surface. These deposits induce the formation of sometimes needle-like lithium metal dendrites, which lead to short circuits and a significant drop in capacity in worst case.
The researchers see a possible solution in the use of single-crystal NCM materials. These showed no loss of capacity even after 200 cycles. Sven Klein, PhD student at MEET Battery Research Center, explains: “In order to achieve high energy density and at the same time a long lifetime, we want to prevent the unwanted interaction of the cathode with the anode in high-voltage LIB cells. We now have very concrete starting approaches for improving high-voltage lithium-ion cells with single-crystal materials, for example.”
Detailed results have been published in the journal “ChemSusChem” by MEET scientists Sven Klein, Peer Bärmann, Thomas Beuse, Dr Tobias Placke as well as Kristina Borzutzki, Johannes Kasnatscheew, Helmholtz Institute Münster, Joop Enno Frerichs, Institute for Physical Chemistry at University of Münster, and Prof. Dr Martin Winter, MEET Battery Research Centre and Helmholtz Institute Münster. The study is embedded in the “Go3” project, which is funded by the German Federal Ministry for Economic Affairs and Energy.

