Parameter of the interface between lithium metal anode and polymer electrolyte decoded
It is one of the key technologies for high-performance batteries of the future: the lithium metal battery (LMB). Designed for instance as solid-state battery with polymer electrolyte, it promises a significantly higher energy density than the currently common lithium-ion battery (LIB). However, it is not yet fully developed for the final commercial breakthrough and is therefore currently used by only a few pilot projects. MEET scientists led by Mengyi Zhang and Martin Kolek have now identified further factors that have a significant influence on the interface between the lithium metal anode and a polymer electrolyte. With their research results, they provide important approaches for further optimising lithium metal technology.
Interface as a challenge
The MEET scientists investigated wetting phenomena and their effects on the electrochemical performance of lithium metal anodes. "Research on interfaces and their characterisation has become increasingly important in recent years," explains Martin Kolek. "This is particularly important because it helps to better understand the processes involved in lithium deposition and dissolution and thus leads to new approaches to solutions".
The surface of the lithium metal anode has microscopically small defects. They occur, for example, during production or processing of the lithium electrode or as a result of lithium deposition and dissolution during battery life. It is therefore all the more important that the electrolyte wets these points, so that the interface is as homogeneous as possible and a large, active surface is created.In this way, overpotentials, which can lead to decomposition of the electrolyte or uncontrolled nucleation, are reduced. Lithium is increasingly deposited inhomogeneously in this process, which in turn can lead to safety risks.
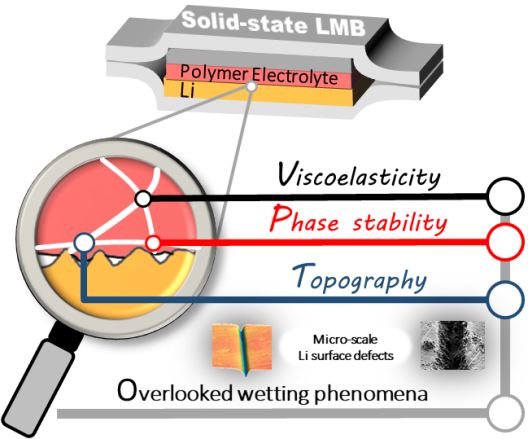
While liquid electrolytes almost completely wet the defect areas, the solid-state electrolyte faces the challenge of forming a homogeneous interface between two solid materials. The MEET scientists have now discovered that the viscoelastic properties of the polymer electrolyte significantly influence the wetting behaviour. Such substances combine the characteristics of liquid and solid substances. Put simply, the more deformable the electrolyte is, the better it can wet defect areas. However, this knowledge is not sufficient to completely understand the wetting behaviour. This is why the scientific team has examined it in greater detail in the study.
Important results for the understanding of the wetting induced electrochemical interface
MEET scientist Mengyi Zhang explains: "In our experiments, we artificially created the defect areas and thus imitated a rough lithium surface. We then varied the curved edges of the surface and analysed their influence on the wetting and deposition behaviour". And these are far-reaching: they influence the nucleation and the electrochemical properties as well as the impedance of the cells. This means that the type of curvature and the quality of its wetting have a decisive influence on the lithium deposition and the formation of the surface layers at the interface between lithium and polymer electrolyte. In this way, they significantly determine the capacity and safety of the battery.
"Further research into surface curvature and its effects on lithium deposition, even under varying conditions, is therefore essential," emphasizes Zhang. The MEET scientist is working on the improvement of (polymer-based) electrolyte systems for LMBs as part of his PhD, thus making an important contribution to research on these battery systems.
Detailed information on the structure of the study and its results have been published in the journal "Angewandte Chemie" by MEET scientists Mengyi Zhang, Jens Becking, Dr. Marian Cristian Stan, Dr. Peter Bieker, Martin Kolek and Prof. Dr. Martin Winter in close cooperation with Arthur Lenoch from the Institute of Physical Chemistry. The Open Access article is available in both German (coming soon) and English.

