



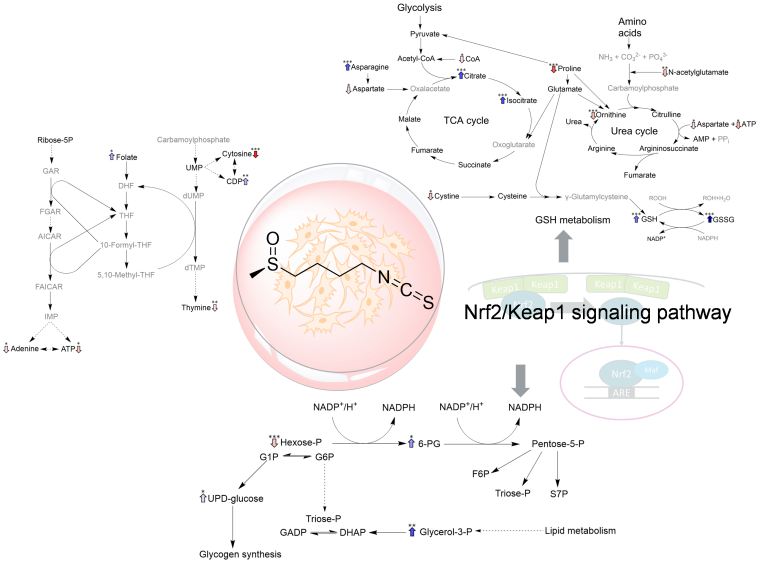
The consumption of vegetables from the cruciferous family (e.g. broccoli) are associated with a healthy diet. Cruciferous vegetables contain sulphureous secondary plant compounds, the glucosinolates, which can be converted to isothiocyanates through food preparation and consumption. Concerning broccoli, the precursor glucoraphanin is converted to the isothiocyanate sulforaphane. Sulforaphane is discussed in the context of health- promoting properties. To gain deeper insights into its mode of action, Nadine Bieß, a doctoral student of the working group of Prof. Dr. Hans-Ulrich Humpf, analyzed the effects of sulforaphane on the cellular metabolism. For this purpose, liver carcinoma cells, which served as model organism, were incubated with sulforaphane and changes in the main metabolic pathways were determined by hydrophilic interaction chromatography with mass spectrometric analysis of more than 100 metabolites. In doing so the methodology was expanded to better detect unstable thiole metabolites.
The results revealed the various effects of sulforaphane on the metabolic pathways. An increase of the antioxidant metabolites was observed and supports the broadly discussed assumption, that sulforaphane stimulates the cellular defense against oxidative stress. Furthermore, influences on the metabolic processes responsible for energy production were detected. Additionally, changes in the amino acids’ metabolism were observed. These results contribute a deeper understanding of the effects of sulforaphane on the cellular metabolism. The results of the study were published in the journal Molecular Nutrition and Food Research (https://doi.org/10.1002/mnfr.70373).

On November 29th, the graduates of the MSc. Food Chemistry together with other graduates of the Master's degree courses in the Faculty of Chemistry and Pharmacy were awarded their certificates. Together with their families and friends, the students had the opportunity to look back on their studies at the festive event and celebrate their achievements.

The prize for the best Master's thesis in Food Chemistry at the University of Münster was also presented during the event. The prize, sponsored by ALS Germany GmbH and endowed with € 1000, was awarded this year to Stephanie Rochau.
The award-winning thesis is entitled "Studies on microbiological processes in activated-carbon filters for drinking water production" and was conducted at the Institute of Molecular Microbiology and Biotechnology under supervision by Prof. Dr. Bodo Philipp, co-supervised by the Institute of Food Chemistry od the University of Muenster.

Mycotoxins are secondary metabolites of moulds and important food contaminants. High exposure to mycotoxins can, for example, increase the risk of cancer or negatively affect child development. By determining individual mycotoxin exposure in humans, these risks can be assessed, correlations with health effects identified and, if necessary, measures to reduce exposure derived. Mycotoxins mostly enter the human body through the consumption of contaminated food, where they are partially metabolised and excreted in the urine. Exposure to mycotoxins can thus be calculated by determining these so-called biomarkers in urine. For statistical analysis, large sets of samples from a population have to be analyzed to calculate reliable exposure data. Therefore, analytical methods for the detection of mycotoxin biomarkers must ensure high sample throughput, i.e. enable numerous analyses to be carried out in the shortest possible time. Due to the usually very low mycotoxin concentrations and the structurally very different mycotoxins, additional analytical challenges arise.
As part of a current research project, Michael Kuhn from Prof. Dr. Hans-Ulrich Humpf's working group developed a new method based on automated solid-phase extraction (online solid phase extraction, online SPE) to combine these requirements. The online SPE was directly coupled with further liquid chromatographic separation and mass spectrometric detection. The urine sample were directly purified by online SPE in several steps and the desired analytes concentrated. This enabled a high sensitivity of the method to be achieved while minimising the manual effort required for sample preparation. A special dilution of the online SPE eluate enabled the measurement of the highly polar analytes deoxynivalenol and deoxynivalenol glucuronides, together with other non-polar mycotoxins. A total of 36 mycotoxins and mycotoxin metabolites were incorporated into the method. The performance of the newly developed method was demonstrated using 50 urine samples from Bangladesh. In addition to analysis using online SPE, the samples were also examined after simple dilution and without further sample preparation (dilute and shoot). Comparison of the results of both methods showed that the online SPE method enabled 304% more positive findings. A total of 15 different mycotoxins and their metabolites were detected in the samples.
Thanks to low detection limits, reduced manual sample preparation and a wide range of analytes, the online SPE approach is a powerful method for reliably estimating mycotoxin exposure. The results were published open access in the Microchemical Journal (https://doi.org/10.1016/j.microc.2025.115821).
The research project was funded by the German Research Foundation (INST 211/1043–1 FUGG).
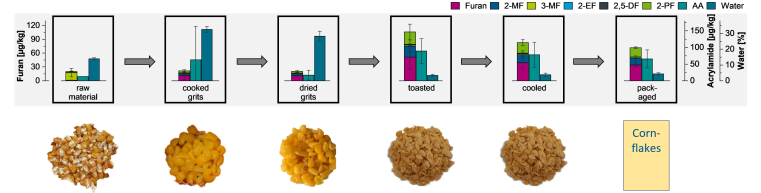
Furans are contaminants that can be formed during the thermal processing of foods. Examples are baking, toasting, frying, or puffing. Modern methods for producing breakfast cereals often involve several cooking or heating steps, which can influence the formation of furans. A cooperative project with various breakfast cereal manufacturers investigated which processes contribute most significantly to the formation of furans and acrylamide, another contaminant monitored. The aim of the project, funded by the Federal Ministry for Economic Affairs and Energy (AIF 21305 N), was to develop new strategies for minimizing the formation of furans based on these investigations.
By analyzing raw materials, intermediate products, and end products of commercial breakfast manufacturing lines, we were able to show how much the individual processing steps affect the formation of these substances and at which points adjustments to the process conditions may be possible. The results of these investigations have now been published in the journal Food Control under the title “Formation of furan, alkylfurans and acrylamide during breakfast cereal manufacturing: Comparison of model experiments with industrial processes.” The formation of furans and acrylamide during cornflakes production is shown here as an example.
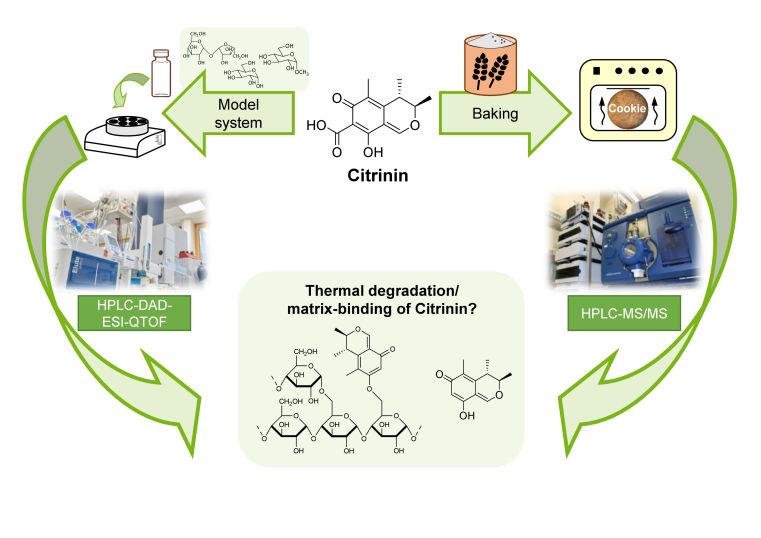
Citrinin is a toxic substance (mycotoxin) produced by mold fungi that occurs in various foods, particularly in grain products. It is formed during plant growth in the field, but mainly during storage. Analysis has shown that the citrinin content in grain is significantly higher than in finished cereal products. While grain cleaning can only partially explain this decrease, it is assumed that citrinin is also degrades during thermal processing such as baking.
As part of her doctoral thesis, Lea Brückner from the working group of Professor Dr. Hans-Ulrich Humpf investigated the reactions of citrinin during thermal food processing. The goal was to understand what happens to citrinin during these processes and whether its "disappearance" is accompanied by a reduction in its toxicity. Following initial studies on the reaction of citrinin with proteins, which were published last year in the journal Mycotoxin Research (https://doi.org/10.1007/s12550-024-00557-y), Lea was now able to demonstrate how citrinin can react with sugar structures. Starting from model compounds, she derived a potential reaction mechanism for the formation of citrinin-sugar conjugates. For transfer to real foods and baking processes, she successfully developed an enzymatic hydrolysis method that enabled the detection of these compounds in actual foods. Furthermore, she investigated influencing factors on the formation of another reaction product of citrinin, decarboxycitrinin. Cell culture experiments also provided initial data on the toxicity of the formed decarboxycitrinin. The results of these studies, as well as the development of the analytical method, have been published open-access in the journal Toxins (https://doi.org/10.3390/toxins17020086).
The research project was funded by the German Research Foundation (HU 730/14-1).
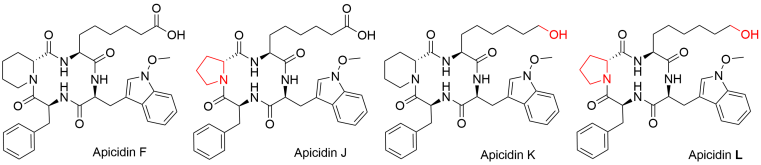
The core structure of apicidins consists of four amino acids linked together in a cyclic configuration. These compounds, produced by various Fusarium species, are best known for their efficacy against the malaria parasite Plasmodium falciparum, which is due to their inhibition of histone deacetylases. This inhibition affects the accessibility of DNA for transcription, thereby influencing multiple cellular signaling pathways.
At the Institute of Food Chemistry, previously research on the pathogenic filamentous fungus Fusarium fujikuroi has been conducted. During that time, the team identified three additional apicidins—Apicidin F, J, and K—following genetic modification of the wild-type strain. Apicidins J and K were found to be structural derivatives of Apicidin F, differing by just one amino acid in their ring structure.
Building on this work, Alica Fischle, a PhD student in Dr. Svetlana Kalinina’s group, has continued the investigation. The study began with an analysis of mutant strains to assess their cultivability and the potential presence of an additional apicidin derivative that incorporates the modifications found in Apicidins J and K. After optimizing the culture conditions, the new substance was extracted, purified, and characterized alongside the known apicidins. Structural analysis using nuclear magnetic resonance spectroscopy (NMR) and high resolution mass spectrometry (HRMS) successfully identified the new tetrapeptide as “Apicidin L”.
Further research evaluated the cytotoxicity of all four apicidins, as well as their biological activity against P. falciparum and their ability to inhibit histone deacetylases. The findings revealed that the structural differences among the apicidins led to varied effects in biological systems. The full results of this study are available as open access in the Journal of Natural Products and Bioprospecting at https://doi.org/10.1007/s13659-024-00473-9. The project also received a poster prize at the ACS Symposium on Biological and Medicinal Chemistry in March 2023, which can be viewed at https://axial.acs.org/biology-and-biological-chemistry/poster-prize-winners-acs-publications-symposium-biological-and-medicinal-chemistry.