High Resolution Cryo Electron Microscopy- Fundamental cellular processes
To this end, cryoEM is one of our key methods. Modern high-end electron microscopes allow the direct observation of biological specimens, e.g. proteins, viruses and large macromolecular complexes, purified or even in their functional cellular environments. Due to recent advances in hardware and software, cryoEM can now resolve features at the atomic level of detail. This provides unique opportunities to obtain essential insights into the functioning of complex macromolecular machines and mechanistic insights into cellular pathways associated with disease and thus develop a strong foundation in designing novel therapeutic treatments.
This leading-edge high-resolution structure elucidation technique is however currently not available in the greater area of Münster! We recently moved to the WWU and one of our first priorities will be to establish a cutting-edge cryoEM facility at the Center for Soft Nanoscience. We are excited about the numerous future opportunities for collaborative research and we are confident that the new infrastructure will set the stage for the development of novel biotechnological and therapeutic approaches.
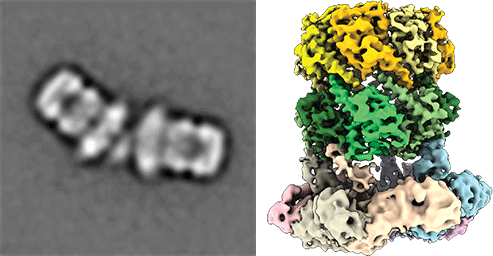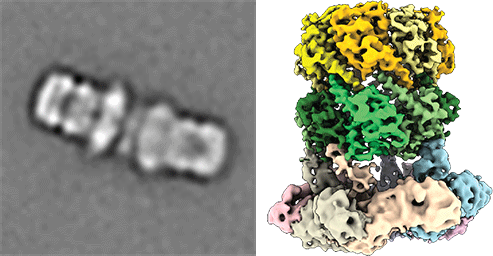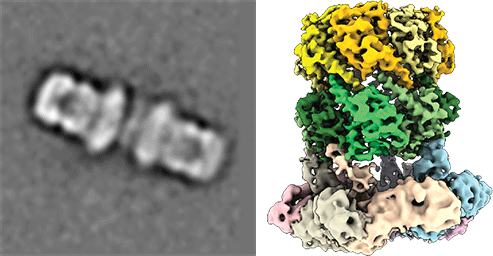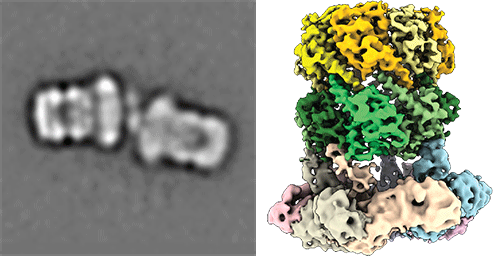
Understanding peroxisomal biogenesis
Our research concentrates on the molecular understanding of peroxisomal biogenesis. Peroxisomes are dynamic small organelles, ubiquitous in nearly all eukaryotes. They are able to carry a plethora of crucial metabolic functions including the β-oxidation of fatty acids and degradation of toxic hydrogen peroxide. The respective enzymes are synthesized on free cytoplasmatic ribosomes and later imported into the peroxisomal lumen by the peroxisomal import machinery. The main characteristics of this process remain however poorly understood. Peroxisomal dysfunction and impaired peroxisomal import result in devastating inborn metabolic disorders, which further emphasizes the importance of understanding these fundamental biological questions.
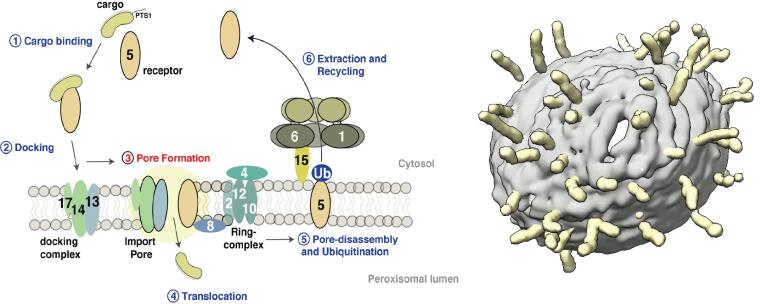
Another focus is on the molecular understanding of the mechanism of pore-forming neurotoxins and AAA-ATPases, which play a key role in protein quality control. We therefore employ protein-biochemistry, biophysical and bioinformatical methods and in particular cryoEM (single particle analysis and cryo electron tomography).
To address our complex and multifaceted central questions, multidisciplinary research, strong synergies and state-of-the-art infrastructure are necessary. For us, the SoN provides in this context an ideal research environment.


