The mechanism of DNA supercoiling, relaxation and deatenation by DNA topoisomerases
People involved: Rahi Adhikari, Airat Gubaev, Jessica Guddorf, Jana Hirsch, Vaibhav Prakash Mhaindarkar, Somenath Roy Chowdhury, Daniela Schlingmeier, Florian Willing
DNA topology
DNA supercoiling impacts key cellular events such as replication, transcription, recombination, and the storage of the genome as chromatin. DNA topoisomerases catalyze the inter-conversion of different DNA topoisomers (reviewed in (1)). This reaction requires DNA cleavage and ligation, which is mediated via trans-esterification reactions involving a conserved tyrosine. Based on structural and mechanistic differences, topoisomerases are grouped into type I and type II enzymes, and A and B subtypes. Type I enzymes cleave one strand of their substrates, and type II enzymes cleave both DNA strands. Enzymes of subtype A form a covalent bond with the 5’-end of the cleaved DNA, while B-type topoisomerases covalently bind the 3’-end(s). For topoisomerases IA and IIA and B, a strand-passage mechanism for DNA supercoiling or relaxation has been postulated.
The type IIA topoisomerases gyrase, topo II and topo IV share a similar architecture, but catalyze different reactions
Type IIA topoisomerases form a symmetric structure with three protein/protein interfaces (gates; Figure 1A). Despite their common architecture, members of this family catalyze different reactions: Eukaryotic topoisomerase II (topo II) catalyzes ATP-dependent DNA relaxation (2), bacterial gyrase mediates the ATP-dependent introduction of negative supercoils (3), and topoisomerase IV (topo IV) mediates ATP-dependent DNA decatenation (4). All of these reactions are believed to occur via strand passage, guided by coordinated opening and closing of the three gates (Figure 1B): First, a double-stranded DNA-segment, the so-called G-segment (for gate), binds at the DNA-gate, and both strands of the DNA are cleaved. ATP-dependent closing of the N-gate then traps a second double-stranded DNA, the T-segment (for transport). DNA-gate opening allows for passage of the T-segment through the gap in the G-segment. The G-segment is religated, and the T-segment leaves the enzyme through the C-gate. Topoisomerase VI (topo VI), a type IIB topoisomerase, lacks the C-gate (Figure 1C) and catalyzes ATP-dependent DNA relaxation by a hitherto uncharacterized two-gate mechanism (5, 6).
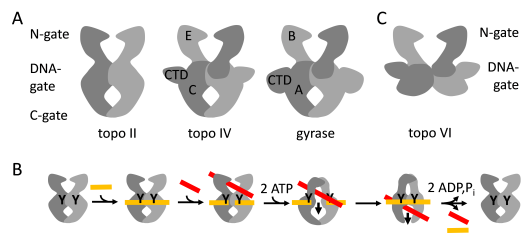
Figure 1: Architecture of type II DNA topoisomerases.
A: Type IIA topoisomerases. Eukaryotic topo II, topo IV and bacterial gyrase share a common architecture with three protein-protein interfaces, termed N-gate, DNA-gate and C-gate. Topo II is a dimer of two Top2 subunits (light and dark gray). Gyrase and topo IV are tetramers formed by two GyrB/ParE subunits (B, E; light and dark gray) corresponding to the N-terminal region of Top2, and two GyrA/ParC subunits (A, C; light and dark gray) corresponding to the C-terminal region of Top2. The GyrA and ParC subunits contain C-terminal domains (CTD) that modulate the interaction of gyrase/topo IV with DNA. B: Scheme of the strand passage mechanism: A T-segment (red) is transported through a gap in the G-segment (orange), generated by double-strand cleavage. Y: catalytic tyrosines. Strand passage is mediated by the coordinated opening of the N-, DNA- and C-gates. C: Topo IV, a type IIB topoisomerase, lacks the C-gate and catalyzes ATP-dependent DNA relaxation using two gates.
We have shown that gyrase undergoes a series of highly coordinated ATP- and DNA-driven conformational changes during its catalytic cycle (7-9). Gyrase only needs one of the two catalytic sitesfor ATP binding and hydrolysis (10), and only one of its CTDs for DNA supercoiling (11). Gyrase containing only a single catalytic tyrosine can also catalyze DNA supercoiling. This reaction must occur in the absence of strand passage, and we have proposed a nicking-closing mechanism for DNA supercoiling based on our experimental data (12). We currently investigate the mechanism of DNA supercoiling, DNA relaxation and DNA decatenation by type IIA topoisomerases.
Reverse gyrase is the only topoisomerase that introduces positive supercoils into DNA
Reverse gyrase is a unique representative of type IA topoisomerases that introduces positive supercoils into DNA at the expense of ATP hydrolysis (13). The enzyme consists of an N-terminal helicase-like domain, fused to a C-terminal topoisomerase I domain. The helicase-like domain contains all helicase signature motifs, but the sequences deviate significantly from the consensus, and no DNA unwinding was determined for the isolated helicase-like domain, or for reverse gyrase. The helicase core is flanked by an N-terminal putative zinc finger, and the second RecA-like domain is interrupted by the so-called latch domain that appears to be involved in inter-domain communication (14-16). From the crystal structure, a strand passage mechanism similar to type IA topoisomerases has been suggested (14) (Figure 2). A conformational change in the helicase-like domain induces a movement of the latch and a concomitant release of the lid. The cleaved DNA strand is covalently attached to the lid domain that swings up to create an opening in the cleaved strand (corresponding to the DNA-gate in gyrase, see Figure 1). Directional passage of the second strand through the gap, followed by re-ligation, leads to positive supercoiling (Figure 2). We have recently mapped the conformation of the helicase-like domain throughout the nucleotide cycle (17), and have shown that the cleft between the RecA domains closes upon binding of DNA and ATP. In contrast to conventional DEAD-box proteins, re-opening already occurs upon ATP hydrolysis, the rate-limiting step in the catalytic cycle (17, 18)). The latch, an insertion in the second RecA domain, is responsible for coupling DNA and ATP binding, and for communication between the helicase-like and the topoisomerase domain (19).
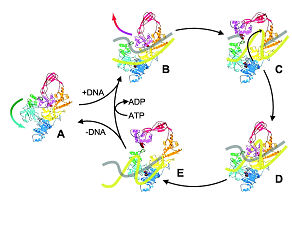
Figure 2: Catalytic cycle of reverse gyrase
Reverse gyrase from A. fulgidus (1GKU, Ref. (14)) comprises a topoisomerase domain (yellow, red, magenta, and orange), and a helicase-like domain containing helicase signature motifs (dark and light blue), with a “latch” domain (green). The catalytic tyrosine is shown in magenta.
(A) Binding of double-stranded DNA (B); cleavage of one strand (C), which remains covalently bound to the active site tyrosine via a phosphoester, and strand passage; re-ligation (D); and release via latch opening (E). Modified after Ref. (14).
References
1. Schoeffler AJ, Berger JM. 2008. Q Rev Biophys 41: 41-101
2. Goto T, Wang JC. 1982. J Biol Chem 257: 5866-72
3. Gellert M, Mizuuchi K, O'Dea MH, Nash HA. 1976. Proc Natl Acad Sci 73: 3872-6
4. Peng H, Marians KJ. 1993. Proc Natl Acad Sci 90: 8571-5
5. Buhler C, Lebbink JH, Bocs C, Ladenstein R, Forterre P. 2001. J Biol Chem 276: 37215-22
6. Corbett KD, Benedetti P, Berger JM. 2007. Nat Struct Mol Biol 14: 611-9
7. Lanz MA, Farhat M, Klostermeier D. 2014. J Biol Chem 289: 12275-85
8. Lanz MA, Klostermeier D. 2012. Nucleic Acids Res 40: 10893-903
9. Lanz MA, Klostermeier D. 2011. Nucleic Acids Res 39: 9681-94
10. Hartmann S, Gubaev A, Klostermeier D. 2017. J Mol Biol 429: 3717-29
11. Stelljes JT, Weidlich, D., Gubaev, A., Klostermeier, D. 2018. Nucleic Acids Res 46(13):6773-6784
12. Gubaev A, Weidlich D, Klostermeier D. 2016. Nucleic Acids Res 44: 10354-66
13. Kikuchi A, Asai K. 1984. Nature 309: 677-81
14. Rodriguez AC, Stock D. 2002. EMBO J 21: 418-26
15. Rodriguez AC. 2002. J Biol Chem 277: 29865-73
16. Rodriguez AC. 2003. Biochemistry 42: 5993-6004
17. del Toro Duany Y, Klostermeier D. 2011. Phys Chem Chem Phys 13: 10009-19
18. del Toro Duany Y, Jungblut SP, Schmidt AS, Klostermeier D. 2008. Nucleic Acids Res 36: 5882-95
19. Ganguly A, del Toro Duany Y, Rudolph MG, Klostermeier D. 2010. Nucleic Acids Res 39: 1789–800.
Hirsch, J., Klostermeier, D. (2021), What makes type IIA topoisomerase a gyrase or a Topo IV?, Nucleic Acids Res., doi: 10.1093/nar/gkab270
Klostermeier, D. (2021) “Towards conformationally-sensitive inhibition of gyrase: Implications of mechanistic insight for the identification and improvement of inhibitors of activities”, for the Molecules Special Issue “DNA Topoisomerases: Structure, Function and Mechanism and Regulation”, Molecules 26(5):1234. doi: 10.3390/molecules26051234.
Klostermeier, D. (2018) Why two? On the role of (a-)symmetry for negative DNA supercoiling by gyrase, Int. J. Mol. Sci. 9(5). pii: E1489. doi: 10.3390/ijms19051489
Hartmann, S., Weidlich, D. & Klostermeier, D. (2016) Investigating the mechanism of negative DNA supercoiling by gyrase using single molecule FRET and confocal microscopy, in “Single molecule Enzymology”, Methods in Enzymology 581:317-351
Lulchev, P. & Klostermeier, D. (2014) Reverse gyrase: recent advances and current understanding of positive DNA supercoiling, Nucl. Acids Res. 42(13):8200-8213
Gubaev, A. & Klostermeier, D. (2014) Single molecule FRET reveals DNA- and nucleotide-induced conformational changes in DNA gyrase preceeding the strand passage reaction, for the DNA repair Special Issue “Single molecule approaches: watching DNA repair one molecule at a time”, DNA repair 16: 23-34
Collins, F., Weisslocker-Schaetzel, M., Klostermeier, D. (2020) A -hairpin is a minimal latch that supports positive supercoiling by reverse gyrase, J. Mol. Biol. 432(16):4762-4771
Weidlich, D., Klostermeier, D. (2020) Functional interactions between gyrase subunits are optimized in a species-specific manner, J. Biol. Chem. 295(8):2299-2312
Stelljes, J., Weidlich, D. & Klostermeier, D. (2018) Gyrase with a single CTD introduces negative supercoils into DNA in steps of two, Nucleic Acids Res., 46(13):6773-6784
Hartmann, S., Gubaev, A., Klostermeier, D. (2017) Binding and hydrolysis of a single ATP is sufficient for negative supercoiling of DNA by gyrase, J. Mol. Biol. 429(23):3717-3729
Gubaev, A., Weidlich, D., Klostermeier, D. (2016) Gyrase with a single catalytic tyrosine can catalyze negative DNA supercoiling by a nicking-closing mechanism, Nucleic Acids Res. 44(21):10354-10366
Rudolph, M.G., Jungblut, S.P., del Toro Duany, Y., Klostermeier, D. (2013) Crystal structures of Thermotoga maritima reverse gyrase: Towards completing the picture for positive DNA supercoiling, Nucleic Acids Res. 41(2):1058-1070
Ganguly, A., del Toro Duany, Y., Klostermeier, D. (2013) Reverse gyrase transiently unwinds double-stranded DNA in an ATP-dependent reaction, J. Mol. Biol. 425(1):32-40
Lanz, M.A. & Klostermeier, D. (2012) The GyrA-box determines the geometry of DNA bound to gyrase and couples DNA binding to the nucleotide cycle, Nucleic Acids Res. 40(21):10893-903
Ganguly, A., Del Toro Duany, Y. & Klostermeier, D. (2011) The latch modulates nucleotide and DNA binding to the helicase-like domain of Thermotoga maritima reverse gyrase and is required for positive DNA supercoiling, Nucleic Acids Res. 39(5), 1789-800
Gubaev, A., Hilbert, M. and Klostermeier, D. (2009) The DNA-gate of B. subtilis gyrase is predominantly in the closed conformation during the supercoiling reaction, Proc. Natl. Acad. Sci. 106, 13278-13283
