The mechanism of RNA unwinding by RNA helicases
People involved: Anirban Chakraborty, Pascal Donsbach, Jessica Guddorf, Óscar Herrera Chacón, Sandy Kokoschka, Linda Krause, Daniela Schlingmeier
DEAD-box helicases mediate local RNA destabilization and unwinding in all processes involving RNA.
Helicases are enzymes that unwind double-stranded nucleic acid in an ATP-dependent reaction (reviewed in ref. (1)). RNA helicases use the energy of ATP hydrolysis to separate RNA duplexes and to remodel RNA structures. They are involved in virtually all processes in RNA metabolism, from transcription, mRNA splicing and editing, transport and translation to RNA degradation (reviewed in ref. (2)). RNA helicases are also required for the structural rearrangement of several RNA/protein complexes, such as ribosomes and spliceosomes (reviewed in refs. (3, 4)). Malfunction of RNA helicases frequently leads to disease (reviewed in ref. (5)).
DEAD-box helicases (reviewed in refs. (6, 7)) are the largest family of RNA helicases. They share a common helicase core that is formed by two RecA domains, connected by a flexible linker (Figure 1). In the absence of RNA or ATP, the core is in the open conformation with the two domains splayed apart. Cooperative binding of RNA and ATP induce a transition to a closed state in which the two RNA binding sites from both domains become aligned, and the active site for ATP hydrolysis is formed (8, 9). Closing of the inter-domain cleft is linked to destabilization of the bound RNA duplex, and thus is a central step for RNA unwinding (8). ATP hydrolysis and phosphate release then reset the helicase core to the open state for subsequent catalytic cycles (10, 11).
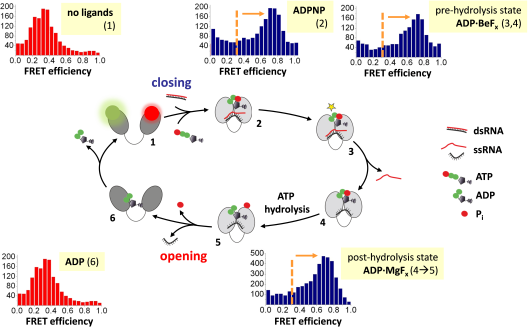
Figure 1: The conformational cycle of the DEAD-box helicase core is linked to duplex destabilization and local RNA unwinding. The helicase core is in an open conformation (low FRET) in the absence of ligands (1; green: donor, red: acceptor). Cooperative binding of ATP and RNA triggers closing of the helicase core (high FRET; 2), followed by a rearrangement to a hydrolysis-competent state (3) from which the first strand of the destabilized duplex dissociates (3à4). The core remains in the closed conformation during ATP hydrolysis (4à5), and re-opens after ATP hydrolysis, upon phosphate and RNA release (5à6). Modified after ref. (12)
In many DEAD-box helicases the helicase core is flanked by N- and C-terminal extensions that contribute to nucleotide binding and hydrolysis (13, 14), binding of RNA (15-19) and protein partners (20), and duplex destabilization and unwinding (20, 21) (reviewed in ref. (22)). We study a number of different helicases to understand the regulation of the helicase core by ancillary domains.
Our contributions (selected):
Donsbach, P., Klostermeier, D. (2021), “Regulation of RNA helicase activities: Principles and case studies, for the Biological Chemistry Special Issue “Structure, mechanism, function and regulation of RNA helicases”, in press
Krause, L., Klostermeier, D. (2021) “Probing RNA helicase Conformational Changes by Single-Molecule FRET Microscopy Probing RNA helicase mechanisms using single-molecule fluorescence methods, for the Methods Mol. Biol. Special Issue “Single molecule approaches: watching DNA repair one molecule at a time”, 2209:119-132
Rudolph, M.G. & Klostermeier D. (2015) When core competence is not enough: functional interplay of the DEAD-box helicase core with ancillary domains and auxiliary factors in RNA binding and unwinding, Biol. Chem. 396(8):849-65
Steimer, L. & Klostermeier, D. (2012) “RNA helicases in infection and disease”, Special Issue on “RNA and Disease” RNA Biology 9(6):751-7
Hilbert, M., Karow, A.R., Klostermeier, D. (2009) The mechanism of ATP-dependent RNA unwinding by DEAD-box proteins, Biological Chemistry, 390 (12):1237-50
Donsbach, P., Yee, B.A., Sanchez-Hevia, D., Berenguer, J., Aigner, S., Yeo, G.W., Klostermeier, D. (2020) The Thermus thermophilus DEAD-box protein Hera is a general RNA binding protein and plays a key role in tRNA metabolism, RNA 26(11):1557-1574
Andreou, A.Z. Harms, U. & Klostermeier, D. (2019) Single-stranded regions modulate conformational dynamics and ATPase activity of eIF4A to optimize 5’-UTR unwinding, Nucleic Acids Res. 47(10):5260-5275
Samatanga, B., Andreou, A.Z. & Klostermeier, D. (2017) Allosteric regulation of helicase core activities of the DEAD-box helicase YxiN by RNA binding to its RNA recognition motif, Nucleic Acids Res. 45(4):1994-2006
Andreou, A.Z., Harms, U. & Klostermeier, D. (2016) eIF4B stimulates eIF4A activities by a direct interaction through its 7-repeats domain, RNA Biol. 14(1):113-123
Harms, U., Andreou, A.Z., Gubaev, A., Klostermeier, D. (2014) eIF4B, eIF4G and RNA regulate eIF4A activity in translation initiation by modulating the eIF4A conformational cycle, Nucleic Acids Res. 42(12):7911-7922
Steimer, L., Wurm, P., Linden, M.H., Rudolph, M.G., Wöhnert, J., Klostermeier, D. (2013) Recognition of two distinct elements in the RNA substrate by the RNA binding domain of the Thermus thermophilus DEAD box helicase Hera, Nucleic Acids Res. 41(12):6259-72
Hilbert, M., Kebbel, F., Gubaev, A., and Klostermeier, D. (2011) eIF4G stimulates the activity of the DEAD box protein eIF4A by a conformational guidance mechanism, Nucleic Acids Res. 39(6), 2260-70
Aregger, R. & Klostermeier, D. (2009) The DEAD-box helicase YxiN maintains a closed conformation during ATP hydrolysis, Biochemistry, 48 (45), 10679-10681
Rudolph, M.G. & Klostermeier, D. (2009) The Thermus thermophilus DEAD box helicase Hera contains a modified RNA recognition motif domain loosely connected to the helicase core, RNA, 15 (11), 1993–2001
Theissen, B., Karow, A.R., Köhler, J., Gubaev, A., Klostermeier, D. (2008) RNA and ATP cooperatively induce a conformational change in a DEAD box helicase, Proc. Natl. Acad. Sci. 105, 548-553
1. Pyle AM. 2008. Annu Rev Biophys 37: 317-36
2. Linder P, Jankowsky E. 2011. Nat Rev Mol Cell Biol 12: 505-16
3. Martin R, Straub AU, Doebele C, Bohnsack MT. 2012. RNA Biol 9: 1173-82
4. Cordin O, Beggs JD. 2013. RNA Biol 10: 83-95
5. Steimer L, Klostermeier D. 2012. RNA Biol 9: 751-71
6. Putnam AA, Jankowsky E. 2013. BBA 1829: 884-93
7. Hilbert M, Karow AR, Klostermeier D. 2009. Biol Chem 390: 1237–50
8. Sengoku T, Nureki O, Nakamura A, Kobayashi S, Yokoyama S. 2006. Cell 125: 287-300
9. Theissen B, Karow AR, Kohler J, Gubaev A, Klostermeier D. 2008. Proc Natl Acad Sci 105: 548-53
10. Aregger R, Klostermeier D. 2009. Biochemistry 48: 10679–81
11. Liu F, Putnam A, Jankowsky E. 2008. Proc Natl Acad Sci 51: 20209-14
12. Harms U, Andreou AZ, Gubaev A, Klostermeier D. 2014. Nucleic Acids Res 42: 7911-22
13. Collins R, Karlberg T, Lehtio L, Schutz P, van den Berg S, et al. 2009. J Biol Chem 284: 10296-300
14. Fan JS, Cheng Z, Zhang J, Noble C, Zhou Z, et al. 2009. J Mol Biol 388: 1-10
15. Linden MH, Hartmann, R.K., Klostermeier, D. . 2008. Nucleic Acids Res. 36: 5800-11
16. Pugh GE, Nicol SM, Fuller-Pace FV. 1999. J Mol Biol 292: 771-8
17. Karginov FV, Caruthers JM, Hu Y, McKay DB, Uhlenbeck OC. 2005. J Biol Chem 280: 35499-505
18. Kossen K, Karginov FV, Uhlenbeck OC. 2002. J Mol Biol 324: 625-36
19. Tijerina P, Bhaskaran H, Russell R. 2006. Proc Natl Acad Sci 103: 16698-703
20. Yan X, Mouillet JF, Ou Q, Sadovsky Y. 2003. Mol Cell Biol 23: 414-23
21. Del Campo M, Lambowitz AM. 2009. Mol Cell 35: 598-609
22. Rudolph MG, Klostermeier D. 2015. Biol Chem 396: 849-65
