Research
Assembly, structure and function of tricellular occluding junctions
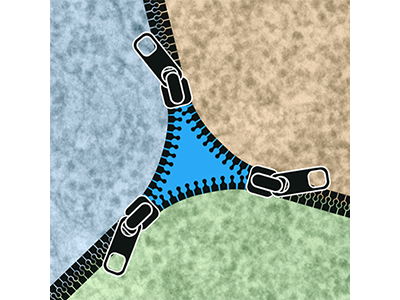
Tricellular junctions (TCJs) provide essential adhesive and occluding functions at epithelial cell vertices and play key roles for tissue integrity and physiology, but how TCJs are assembled and maintained is only beginning to be understood. We identified the transmembrane protein Anakonda (Aka) as a core TCJ component essential for epithelial barrier function. Aka interacts with the tetraspan proteolipid protein M6 to recruit the Neuroligin-like protein Gliotactin (Gli) to cell vertices. To understand how the paracellular diffusion barrier is assembled at TCJs, we use high-resolution imaging, genetics, biochemistry, and structural biology approaches.
Regulation of paracellular permeability through remodeling of cell vertices
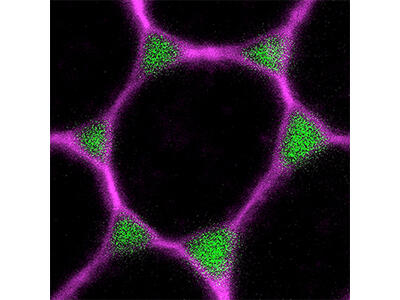
Cell vertices in the follicular epithelium open transiently to allow paracellular passage of fat body-derived yolk precursors (lipids and proteins) for uptake by the oocyte. We established this process, referred to as follicular patency, as a model to investigate how adhesion and actomyosin contractility are remodeled at cell vertices to control epithelial permeability. To address these questions, we combine in vivo experiments with an ex vivo culture system that enables high-resolution imaging and acute manipulation of living follicles using pharmacological and optogenetic tools. RNA-Seq experiments are used to identify genes involved in the regulation of TCJ remodeling during patency.
Acute manipulation and visualization of intracellular transport
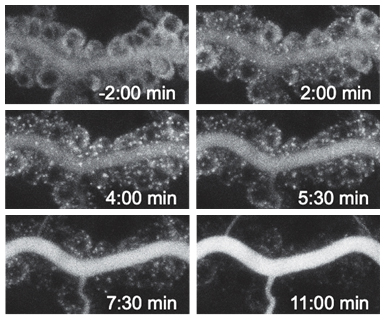
To enable acute manipulation and real-time visualization of membrane trafficking in intact living animals, we adapted the Retention using Selective Hooks (RUSH) system for use in Drosophila. We showed that secreted, transmembrane, and GPI-linked proteins tagged with a Streptavidin-binding peptide (SBP or StrepII) and a fluorophore are efficiently retained in the ER by Streptavidin “hook” proteins. ER retention is rapidly reversed upon addition of biotin, allowing to synchronize intracellular trafficking and to release cargo proteins with high temporal precision. The system enables control of endogenous or overexpressed proteins and is applicable to embryos, larvae and adults, as well to cultured organs ex vivo. Furthermore, hook-induced ER retention can be employed to deplete and acutely restore secretory protein function in a tissue-specific manner. We use RUSH to analyze the kinetics of ER exit and apical secretion, and the dynamics of tricellular junction assembly in epithelia.
