Polyelectrolyte self-assembly
The adsorption of organic molecules to interfaces is offering a variety of possibilities for the design of novel materials with structures defined on a nanometer scale.
So-called "self-assembly"-processes, employing the self-organization of molecules at interfaces as a result of for example hydrophobic or electrostatic interactions can be employed to produce layered materials.
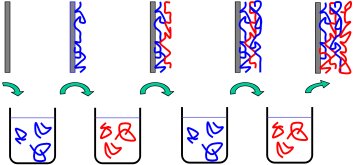
Alternating dip-coating yields polyelectrolyte multilayers© Prof. Dr. Schönhoff Multilayer formation
In this context we are employing polyelectrolyte adsorption, driven by electrostatic interaction. Multiple adsorption steps, alternating between polycations and polyanions yield so-called polyelectrolyte multilayers. Applying a large number of repetitions is a simple way of forming a material with a large variability concerning different molecular constituents.
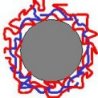
Multilayer-coated particle© Prof. Dr. Schönhoff PARTICLE COATING
PEMs can even be formed on colloidal particles as templates instead of planar substrates. In this case, the excess polyelectrolyte has to be removed by centrifugation or ultrafiltration.
The development of PEM- coating procedures for particles is a major achievement, since it allows to prepare dispersions with a large surface area. In such samples, PEMs can be studied by volume techniques which require a sufficient amount of compound, such as for example NMR or DSC.

Hollow microcapsule formation by template dissolution© Prof. Dr. Schönhoff After coating of colloidal particles the template itself can be removed employing suitable chemical procedures (for example a dissolution or degradation in acidic conditions). Provided that the PEM shell is stable against that treatment, a hollow polymeric capsule is remaining. Such hollow containers are interesting objects for applications such as encapsulation and the controlled release of active compounds. Therefore, the permeability and mechanisms to control it are of great interest.
Why are we interested in these materials?
A number of potential applications make PEMs very interesting. Apart from pure charged homopolymers, a variety of additional components with defined functionalities can be employed in the layer formation, such as for example proteins, enzymes, inorganic nanoparticles, or functional polymers sensitive to external stimuli (pH, temperature). This leads to new possibilities of controlled material design on the smallest possible length scale available with molecular arrangements.
In our group, we employ functional components such as for example gold nanoparticles or guest-host systems in polyelectrolyte multilayers. We employ synthetic or bio-polymers, and we optimize our systems towards applications such as molecular imprinting, pH triggered release or ion conduction.
For any kind of application, however, a basic understanding of the principles of self-organized layer formation is essential. Therefore we also investigate fundamental mechanisms of layer formation, for example interdiffusion processes, which may change the growth law from a linear to an exponential one.
Selected publications:Movilli, J.; Choudhury, S.S.; Schönhoff, M.; Huskens, J.
Enhancement of Probe Density in DNA Sensing by Tuning the Exponential Growth Regime of Polyelectrolyte Multilayers
Chem. Mater. 2020, 32, 9155-9166.
https://dx.doi.org/10.1021/acs.chemmater.0c02454Schlicke, J.; Hoffmann, K.; Lorenz, M.; Schönhoff, M.; Cramer, C.
Ionic Conductivity Enhancement of Polyelectrolyte Multilayers by Variation of Charge Balance
J. Phys. Chem. C 2020, 124 (31), 16773–16783.
https://doi.org/10.1021/acs.jpcc.0c03043Parveen, N.; Jana, P. K.; Schönhoff, M.
Viscoelastic Properties of Polyelectrolyte Multilayers Swollen with Ionic Liquid Solutions
Polymers 2019, 11(8), 1285.
https://doi.org/10.3390/polym11081285Ostendorf, A.; Schönhoff, M.; Cramer, C.
Ionic conductivity of solid polyelectrolyte complexes with varying water content: application of the dynamic structure model
Phys. Chem. Chem. Phys. 2019, 21(14), 7321-7329.
https://doi.org/10.1039/c8cp05853aBütergerds, D., Cramer, C., Schönhoff, M.
pH-dependent growth laws and vicoelastic parameters of poly-L-lysine/ hyaluronic acid multilayers
Adv. Mat. Interf. 2017, 4(1), 1600592
https://doi.org/10.1002/admi.201600592
Nicolas, H., Yuan, B., Zhang, X., Schönhoff, M.
Cucurbit[8]uril-Containing Multilayer Films for the Photocontrolled Binding and Release of a guest Molecule
Langmuir 2016, 32, 2410-2418
Ostendorf, A., Cramer, C., Decher, G., Schönhoff, M.
https://pubs.acs.org/doi/abs/10.1021/acs.langmuir.6b00128
Humidity-Tunable Electronic Conductivity of Polyelectrolyte Multilayers Containing Gold Nanoparticles
J. Phys. Chem. C 2015, 119(17), 9543-9549
https://doi.org/10.1021/jp5127706
Further reading about Polyelectrolyte multilayers:
Schönhoff, M.
Self-Assembled Polyelectrolyte Multilayers
Current Opinion in Coll. Interf. Sci. 2003 , 8(1), 86-95.Schönhoff, M.
Layered Polyelectrolyte Complexes: Physics of Formation and Molecular Properties
J. Phys.: Cond. Matter 2003, 15(49), R1781-R1808.
