Introduction
Plants must constantly respond to a wide range of signals, including stresses, in order to coordinate their development and survival within a dynamic environment. One way in which this is achieved is through chemical modifications of proteins, allowing flexible and rapid changes of the proteome to alter cellular and physiological outputs. Protein acetylation is one such modification, which occurs on the N-termini (Nt) and internal lysines (K) of many proteins (Fig. 1).
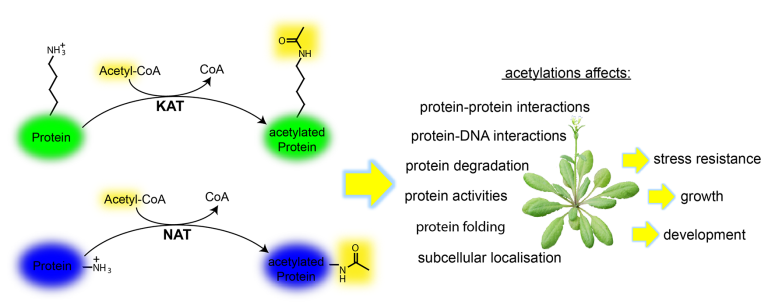
Although, protein acetylation is a prevalent modification, our knowledge of the regulation, specificity and plasticity, and its downstream functional consequences on protein activity and physiology are just emerging, especially in plants.