RESEARCH - Henning Mootz
Protein chemistry / biotechnology
Technology development for protein semisynthesis and protein functional control
The ability to site-selectively incorporate a synthetic moiety like a biophysical probe, an unnatural amino acid, or a defined posttranslational modification into a protein of interest opens up a variety of research avenues to interrogate protein structure and function that cannot be achieved by standard molecular biology approaches. Techniques for protein chemical modification have been around for a long time, however, new chemical reactions, sophisticated molecular biology approaches, and combinations thereof have taken protein chemistry to a new level in recent years.
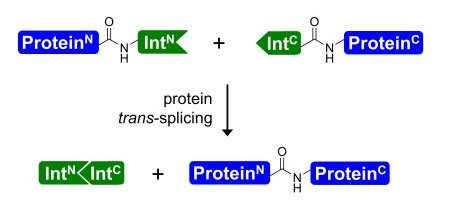
We are interested in exploiting the power of the protein trans-splicing reaction catalyzed by split inteins for the selective chemical modification of proteins. Split intein fragments, intein-N and intein-C, link their fused sequences in a self-processing reaction with a native peptide bond and excise themselves during this process (see Figure 1)(1). They thus provide a means to link, in principle, any two polypeptide sequences, recombinant or synthetic (see Figures 2 and 3). Protein trans-splicing can be used to reconstitute a protein from two inactive fragments, to link a short (labeled or modified) tag to a protein or generally to assemble a single polypeptide chain from two completely unrelated sequences that can also be of different origin, i.e., bacterial vs. mammalian expression systems or recombinant vs. chemical synthesis.
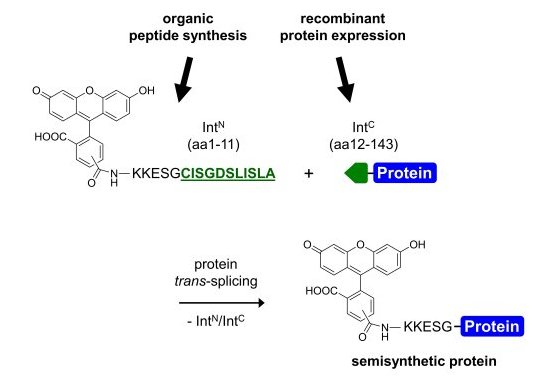
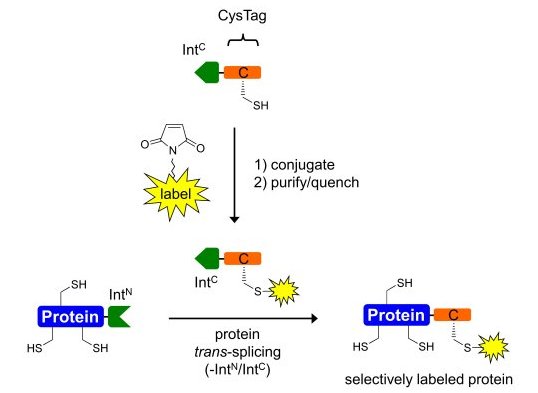
The protein trans-splicing reaction offers several unique advantages over other chemical or enzymatic ligation approaches. For example, due to the high affinity of the intein fragments the reaction can be carried out at low reactant concentrations, it can be performed in complex mixtures like cell extracts or inside living cells, and no functional groups are required. However, it remains a challenge to identify inteins with better properties regarding the splicing efficiency, solubility, fragment sizes, and sequence tolerance for each particular application. A more detailed understanding of the protein splicing mechanism is also important to achieve these goals.
Over the years, we have identified, characterized and further engineered several naturally split inteins, mostly in collaboration with Prof. Dr. Shmuel Pietrokovski (Weizmann Institute). We have demonstrated their utility for various protein engineering applications. Among these intein were the most widely used Gp41-1 and Npu DnaE split inteins with exceptional protein trans-splicing rates (Carvajal-Vallejos et al., 2012; Böcker et al., 2019; Zettler et al. 2009). We also have discovered the first atypically split inteins with a short N-terminal intein fragment that is amenable to solid-phase peptide synthesis (Thiel et al., 2014; Bachmann and Mootz, 2015; Pasch et al., 2023). Such atypical split sites were previously artificially introduced into continuous cis-inteins (Ludwig et al., 2006; Appleby-Tagoe et al., 2011), but usually left the engineered intein fragments more vulnerable to misfolding. More recently, we have reported the first cysteine-less split inteins, both with the typical fragment sizes in case of the Aes123 PolB1 intein (Bhagawati et al., 2019) and with an atypical split site in case of the PolB16 OarG intein (Pasch et al., 2023). Cysteine-less split inteins are of particular interest because without the oxidation-prone catalytic cysteines they can splice under oxidizing conditions, e.g. in the extracellular environment, on the surface of cells (Bhagawati et al., 2019) and in the strict absence of any reducing agents, which is, for example, useful to leave any disulfide bonds in the protein extein sequences intact. We have demonstrated the practical usefulness of this fact with the preparation of various antibody and nanobody conjugates (Pasch et al. 2024; Bhagawati et al., 2019)
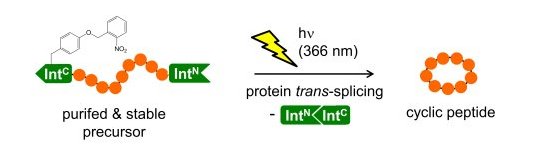
Since protein splicing involves a significant structural change of the fused peptide or protein sequences, an artificial conditional control of this reaction offers exciting opportunities to link to peptide or protein function. For example, we have designed a photocaged, synthetic intein fragment that can be used to trigger a split-intein mediated cleavage reaction by administration of UV-light. By releasing the active form of a coagulase in this way we were able allosterically induce thrombin activity in human blood plasma (Binschik et al, 2011). In another approach we introduced a photocaged amino acid into a fully genetically encoded intein (Figure 4). With this system the protein splicing reaction and the formation of cyclic peptides could be turned on with light (Böcker et al., 2015).
Selected publications
Pasch, T., Bäumer, N., Bäumer, S., Buchholz, F., Mootz, H. D.
Towards Targeted Cas9 (CRISPR-Cas) Delivery: Preparation of IgG Antibody-Cas9 Conjugates Using a Split Intein.
J. Pept. Sci., 2024, online, doi: 10.1002/psc.3592.
Pasch, T., Schröder, A., Kattelmann, S., Eisenstein, M., Pietrokovski, S., Kümmel, D., Mootz, H. D.
Structural and biochemical analysis of a novel atypically split intein reveals a conserved histidine specific to cysteine-less inteins.
Chem. Sci., 2023, 14, 5204-5213, doi: 10.1039/d3sc01200j..
Palei, S., Mootz, H. D.
Semisynthetic head-to-tail cyclized peptides obtained by combining protein trans-splicing and intramolecular expressed protein ligation.
Chem. Comm., 2021, 57, 4194-4197, doi: 10.1039/D1CC00449B.
Hoffmann, S., Terhorst, M. E., Singh, R. K., Kümmel, D., Pietrokovski, S.,* Mootz, H. D.*
Biochemical and structural characterization of an unusual and naturally split class 3 intein.
ChemBioChem, 2021, 22, 364-373, doi: cbic.202000509.
Bhagawati, M., Hoffmann, S., Höffgen, K. S., Piehler, J., Busch, K. B., Mootz, H. D.
In cellulo protein semi-synthesis from endogenous and exogenous fragments using the ultra-fast split Gp41-1 intein.
Angew. Chem. Int. Ed., 2020, 59, 21007-21015, doi: 10.1002/anie.202006822.
Bhagawati, M., Terhorst, T. M. E., Füsser, F., Hoffmann, S., Pasch, T., Pietrokovski, S.,* Mootz, H. D.*
A mesophilic cysteine-less split intein for protein trans-splicing applications under oxidizing conditions.
Proc. Natl. Acad. Sci. U S A, 2019, 116, 22164-22172, doi: 10.1073/pnas.1909825116.
Böcker, J. K., Dörner, W., Mootz. H. D.
Light-control of the ultra-fast Gp41-1 split intein with preserved stability of a genetically encoded photo-caged amino acid in bacterial cells.
Chem. Comm. (Camb), 2019, 55, 1287-1290, doi: 10.1039/c8cc09204d.
Palei, S., Becher, K. S., Nienberg, C., Jose, J., Mootz, H. D.
Bacterial cell surface display of semisynthetic cyclic peptides.
ChemBioChem., 2019, 20, 72-77, doi: 10.1002/cbic.201800552.
Friedel, K., Popp, M., Matern, J. C. J., Gazdag, E. M., Thiel, I. V., Volkmann, G., Blankenfeldt, W., Mootz, H. D.
A functional interplay between intein and extein sequence in protein splicing compensates for the essential block B histidine.
Chem. Sci., 2019, 10, 239-251, doi: 10.1039/C8SC01074A.
Di Ventura, B., Mootz, H. D.
Switchable inteins for conditional protein splicing.
Biol. Chem., 2019, 400, 467-475, doi: 10.1515/hsz-2018-0309.
Matern, J. C. J., Friedel, K., Binschik, J., Becher, K.-S., Yilmaz, Z., Mootz, H. D.
Altered coordination of individual catalytic steps in different and evolved inteins reveals kinetic plasticity of the protein splicing pathway.
J. Am. Chem. Soc., 2018, 140, 11267-11275. doi: 10.1021/jacs.8b04794.
Bachmann, A.-L., Mootz, H. D.
An Unprecedented Combination of Serine and Cysteine Nucleophiles in a Split Intein with an Atypical Split Site.
J. Biol. Chem., 2015, 290, 28792-28804. DOI: 10.1074/jbc.M115.677237.
Böcker, J. K., Friedel, K., Matern, J. C., Bachmann, A.-L., Mootz, H. D.
Generation of a genetically encoded, photoactivatable intein for the controlled production of cyclic peptides.
Angew. Chem. Int. Ed., 2015, 54, 2116-2120. DOI: 10.1002/anie.201409848.
Thiel, I. V., Volkmann, G., Pietrokovski, S., Mootz, H. D.
An atypical naturally split intein engineered for highly efficient protein labeling.
Angew. Chem. Int. Ed., 2014, 53, 1306-1310.
Binschik, J., Mootz, H. D.
Chemical Bypass of Intein-Catalyzed N-S-Acyl Shift in Protein Splicing.
Angew. Chem. Int. Ed., 2013, 52, 4260-4264.
Volkmann, G., Mootz, H. D.
Recent progress in intein research: from mechanism to directed evolution and applications.
Cell. Mol. Life Sci. 2013, 70, 1185-1206.
Carvajal-Vallejos, P., Pallissé, R., Mootz, H. D., Schmidt, S. R.
Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources.
J. Biol. Chem., 2012, 287, 28686-28696.
Appleby-Tagoe, J. H., Thiel, I. V., Wang, Y., Wang, Y., Mootz, H. D., Liu, X.-Q.
Highly efficient and more general cis- and trans-splicing inteins through sequential directed evolution.
J. Biol. Chem., 2011, 286, 34440-34447.
Binschik, J., Zettler, J., Mootz, H. D.
Photocontrol of protein activity mediated by the cleavage reaction of a split intein. Angew. Chem. Int. Ed., 2011, 50, 3249-3252.
Selected as a hot paper by Angew. Chem..
Dhar, T., Mootz, H. D.
Modification of transmembrane and GPI-anchored proteins on living cells by efficient protein trans-splicing using the Npu DnaE intein.
Chem. Commun., 2011, 47, 3063-3065.
Featured on the inside cover of Chem. Commun..
Mootz, H. D.
Split inteins as versatile tools for protein semisynthesis.
ChemBioChem 2009, 10, 2579-2589.
Zettler, J., Schütz, V., Mootz, H. D.
The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans-splicing reaction.
FEBS Lett. 2009, 583, 909-914.
Kurpiers, T., and Mootz, H. D.
Regioselective cysteine bioconjugation by appending a modified cysteine tag to a protein using protein splicing in trans.
Angew. Chem. Int. Ed. 2007, 46, 5234-5237.
Ludwig, C., Pfeiff, M., Linne, U., and Mootz, H. D.
Ligation of a synthetic peptide to the N-terminus of a recombinant protein using semi-synthetic protein trans-splicing.
Angew. Chem. Int. Ed. 2006, 45, 5218-522.
Last updated 04/2024