Research concept
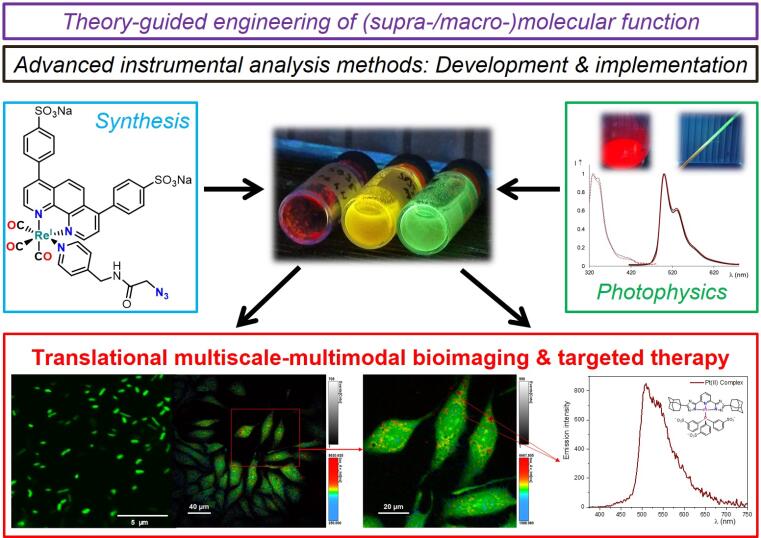
The Strassert Lab is devoted to the theory-guided design and organic synthesis of functional ligands for main-group elements as well as for d- and f-block cations across the periodic table while exploring diverse binding modalities, preparative strategies, redox properties, relativistic and spin effects. With these tools, coordination compounds are engineered for the optical manipulation of structural features and dynamic processes in bio-(macro)molecules and living systems towards tailored probes for multiscale-multimodal bio-imaging, acting simultaneously as contrast agents for electron microscopy or as luminescent markers. Hence, (spectro)electrochemical techniques and time-resolved multiphoton (micro)spectroscopic methods are developed to implement these new entities towards high-resolution optical microscopy. Another goal is the MRI, photoacoustic or radioactive labeling and the targeted ROS-mediated inactivation of antibiotic-resistant pathogens and neoplastic cells by implementing custom-made NIR absorbers for in vivo therapy and diagnostics. The research efforts on molecular photosensitizers and emitters is also oriented towards printable devices employing soft architectures and solution-processable fluorophores or phosphors by unraveling their coupling with (semi)conductive surfaces for (oxygen) sensing and (electro/chemi)luminescence. The quantitative understanding of (macro/supra)molecular function and structure-property relationships are also relevant for opto(bio)electronics as well as for (photoinduced) charge separation processes; thus, they will be also useful for applications in (photo)(bio)catalysis and for the integration of hybrid devices.
Selected publications co-authored by Prof. C. A. Strassert (related to the research concept)
30. Site-specific covalent metalation of DNA oligonucleotides with phosphorescent platinum(II) complexes. Boisten, F. et al., Chemical Science 2023, 14, 2399
29. Room-temperature phosphorescence from Pd(II) and Pt(II) complexes as supramolecular luminophores: The role of self-assembly, metal-metal interactions, spin-orbit coupling and ligand-field splitting. Theiss, T. et al., Journal of the American Chemical Society 2023, 145, 3937
28. Facile modification of phosphole-based aggregation-induced emission luminogens with sulfonyl isocyanates. König, N. et al., Chemical Science 2023, 14, 2267
27. Electrostatic anti‑CD33‑antibody–protamine nanocarriers as platform for a targeted treatment of acute myeloid leukemia. Bäumer, N. et al., Journal of Hematology & Oncology 2022, 15, 171
26. Genetically encoded dual fluorophore reporters for graded oxygen-sensing in light microscopy. Bauer, N. et al., Biosensors and Bioelectronics 2023, 221, 114917
25. Luminescence and Length Control in Nonchelated d8-Metallosupramolecular Polymers through Metal-Metal Interactions. Matern, J. et al., Angewandte Chemie International Edition 2022, 61, e202208436
24. Monoanionic C^N^N Luminophores and Monodentate C-Donor Co-Ligands for Phosphorescent Pt(II) Complexes: A Case Study Involving Their Photophysics and Cytotoxicity. Maisuls, I. et al., Inorganic Chemistry 2022, 61, 9195.
23. Targeted siRNA nanocarrier: a platform technology for cancer treatment. Bäumer, N. et al., Oncogene 2022, 41, 2210
22. Mapping the regioisomeric space and visible color range of purely organic dual emitters with ultralong phosphorescence components: From violet to red towards pure white-light. Roy, B. et al., Angewandte Chemie International Edition 2022, 61, e202111805
21. AIE-Active Difluoroboron Complexes with N,O-Bidentate Ligands: Rapid Construction by Copper-Catalyzed. C−H Activation. Tan, G. et al., Advanced Science 2021, 8, 2101814
20. Conjugated Pt(II) Complexes as Luminescence-Switch-On Reporters Addressing the Microenvironment of Bacterial Biofilms. Maisuls, I. et al., Inorganic Chemistry 2021, 60, 11058
19. Intermolecular Interactions and Self-Assembly in Pt(II) Complex−Nanoclay Hybrids as Luminescent Reporters for Spectrally Resolved PLIM. Gangadharappa, S. C. et al., Journal of Physical Chemistry C 2021, 125, 5739
18. Ligand-controlled and nanoconfinement-boosted luminescence employing Pt(II) and Pd(II) complexes: from color-tunable aggregation-enhanced dual emitters towards self-referenced oxygen reporters. Maisuls, I. et al., Chemical Science 2021, 12, 3270
17. Compensation of Hybridization Defects in Phosphorescent Complexes with Pnictogen-Based Ligands - A Structural, Photophysical, and Theoretical Case-Study with Predictive Character. Gangadharappa, S. C. et al., Journal of the American Chemical Society 2020, 142, 21353
16. Synthesis of Small-Molecule Fluorescent Probes for the In Vitro Imaging of Calcium-Activated Potassium Channel KCa3.1. Brömmel, K. et al., Angewandte Chemie International Edition 2020, 59, 8277
15. A platinum-doped dendrimer as a phosphorescent label for bacteria in two-photon excitation microscopy. Molina Cabeza, N. et al., E. ACS Omega 2019, 4, 13027
14. On-Surface Reactive Planarization of Pt(II) Complexes. Ren, J. et al., Angewandte Chemie International Edition 2019, 58, 15396
13. Phosphorescent Pt(II) complexes spatially arrayed in micellar polymeric nanoparticles providing dual readout for multimodal imaging. Proetto, M. T. et al., Chemical Communications 2019, 55, 501
12. Towards Optimized Naphthalocyanines as Sonochromes for Photoacoustic Imaging in vivo. Duffy, M. J. et al., Photoacoustics 2018, 9, 49
11. Modification of the Potential Landscape of Molecular Rotors on Au(111) by the Presence of an STM Tip. Lu, H. et al., Nano Letters 2018, 18, 4704
10. Oxygen-Insensitive Aggregates of Pt(II) Complexes as Phosphorescent Labels of Proteins with Luminescence Lifetime-Based Readouts. Delcanale, P. et al., ACS Applied Materials & Interfaces 2018, 10, 24361
9. Quantitative assessment of intermolecular interactions by atomic force microscopy imaging using copper oxide tips. Mönig, H. et al., Nature Nanotechnology 2018, 13, 371
8. Toward Tunable Electroluminescent Devices by Correlating Function and Submolecular Structure in 3D Crystals, 2D-Confined Monolayers, and Dimers. Wilde, S. et al., ACS Applied Materials & Interfaces 2018, 10, 22460
7. Labeling and Selective Inactivation of Gram-Positive Bacteria Employing Bimodal Photoprobes with Dual Readouts. Galstyan, A. et al., Chemistry a European Journal 2016, 22, 5243
6. Silicon(IV) Phthalocyanine-Decorated Cyclodextrin Vesicles as a Self-Assembled Phototherapeutic Agent against MRSA. Galstyan, A. et al., ACS Applied Materials & Interfaces 2016, 8, 12631
5. Spatiotemporally Resolved Tracking of Bacterial Responses to ROS-Mediated Damage at the Single-Cell Level with Quantitative Functional Microscopy. Barroso Peña, A. et al., ACS Applied Materials & Interfaces 2016, 8, 15046
4. Selective inactivation of resistant Gram-positive pathogens with a light-driven hybrid nanomaterial. Grüner, M. C.; et al., ACS Applied Materials & Interfaces 2015, 7, 20965
3. Photofunctional surfaces for quantitative fluorescence microscopy: Monitoring the effects of photogenerated ROS at single cell level with spatiotemporal resolution. Stegemann, L. et al., ACS Applied Materials & Interfaces 2015, 7, 5944
2. Scanning-Tunneling-Spectroscopy-Directed Design of Tailored Deep-Blue Emitters. Sanning, J. et al., Angewandte Chemie International Edition 2015, 54, 786
1. Unraveling orbital hybridization of triplet emitters at the metal-organic interface. Ewen, P. et al., Physical Review Letters 2013, 111, 267401
