MEET Researchers Decode Reaction Mechanisms During Interphase Formation in Lithium Batteries
Understanding all processes and reactions inside the lithium-ion battery in detail is essential for its further development. Especially interphases, which strongly influence the performance, safety and service life of energy storage systems, are still insufficiently investigated. They are particularly fragile and vulnerable to common methods for sample preparation. A team of MEET Battery Research Center led by scientist Marco Leißing has now used isotope labelling and gas chromatography to analyse the reaction pathways during formation. The researchers were thus not only able to confirm known reaction equations during interphase formation, but also provide new finding about the decomposition product methane.
Basic Approach for a Better Understanding of Interphase Formation
"The direct analysis of interphases and their formation is only possible to a very limited degree. Therefore, indirect methods are essential to draw conclusions from the occurring reactions and mechanisms," explains MEET PhD student Marco Leißing. "With our analysis, we provide a new, fundamental approach to investigate decomposition reactions during interphase formation."
The scientists focused on gaseous decomposition products, which are formed parallel to the interphase. Using gas chromatography, they were able to indirectly draw conclusions from the formation mechanisms in the cells. For this purpose, the researchers isotopically labelled the solvents as a component of the electrolyte. Since the solvents significantly influence the formation of interphases, the team was able to observe their path through the cells. "We can thus assign the decomposition products to their origin," says Leißing.
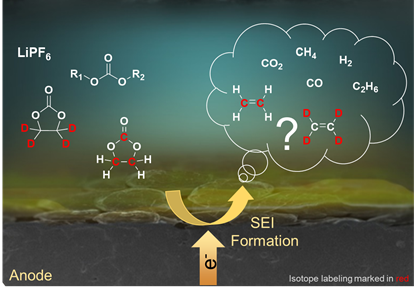
Complex Reactions Lead to the Decomposition Product Methane
Based on their investigations, the MEET researchers confirmed reaction equations from the literature, but also provided new understandings of the decomposition product methane (CH4). "The reactions that lead to the formation of methane are more complex than previously assumed," explains Leißing. Carbon and hydrogen atoms involved come from different parts of the electrolyte. The reaction pathways must now be identified more precisely in further research.
MEET scientists Marco Leißing, Christoph Peschel, Dr Fabian Horsthemke, Dr Simon Wiemers-Meyer, Prof. Dr Martin Winter and Dr Sascha Nowak have published the complete results of their study as an open access article in the scientific journal "Batteries & Supercaps".

