Stress leads to an internal reorganization of the body’s cells
A stressful job, trouble with the children and a near-empty bank account. When everything starts to get too much again, it can help to make a fresh start. If cells are under a lot of stress, for example as a result of injuries, they also undergo a fundamental reorganization. It’s all about their cytoskeleton, which again and again forms new structures from many individual components in a highly flexible way, for example in order to support the cell or to transport molecular charges. A team headed by Prof. Roland Wedlich-Söldner at the Cells-in-Motion Cluster of Excellence at Münster University were able to shed light on this process in detail. The researchers showed that the “new beginning” undergone by the cells is important for repair and wound-healing processes, and that it might play a role too in cell migration, cancer and inflammations. The article has now been published in the journal eLife.
When cells go back to “start“, they pull a structural protein from the cytoskeleton – actin – into their interior. The background to this is that damage to the outer membrane, or other disruptions, can put cells under pressure, and this is often manifested via a universal stress signal. A large quantity of calcium then flows into the cell in a short time. “We can see under the microscope that that has enormous effects on the cytoskeleton,” says Roland Wedlich-Söldner. “What’s particularly affected by this are the so-called actin filaments, which form a tight network on the inside of the cell membrane. This network supports the cell and preserves its outer form.” Right after the calcium has flowed in, these outposts are withdrawn and deposited near the cell nucleus. The researchers call this process “calcium-mediated actin reset” (CaAR).
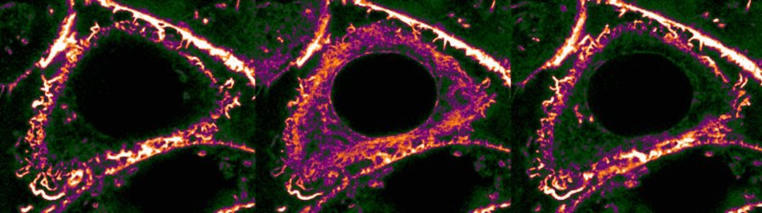
There is one factor that is decisive for this withdrawal – as the researchers were able to demonstrate: the protein INF2. It supervises both the superfast dismantling of the actin network and the formation of the new filaments – thread-like structures in the interior of the cell. The redistribution does not, however last long, because after just a few minutes the calcium has been pumped back out of the cell and the actin network is built up again on the inside of the surrounding cell membrane. So all for nothing, then? “What is decisive is that CaAR allows the cells to restructure the sensitive area near the cell membrane in a very short time, and drastically, as a response to stress,” says Roland Wedlich-Söldner. This means that, in a flash, the structures of the cytoskeleton could be adapted to the new requirements in any stress situation – with far-reaching consequences in the cell.
In this way, the researchers were able to show that the dramatic reconstruction of the actin network can modify the activity of certain genes. The short-term response to stress can lead to long-term effects in the behaviour of the cell, for example in its movements. The researchers also assume that it has an exceptionally wide-ranging importance, because the results of the study – which were confirmed in many different mammal cells – show that CaAR has a role to play in repair processes and in wound healing. The results also suggest that, in the case of stress, calcium might influence the organization of actin and thus the form and the movement of cells, for example in inflammation and cancer. “In further studies we now want to examine whether CaAR plays any role – and, if so, which,” says Roland Wedlich-Söldner.
The work was supported by the Cells-in-Motion Cluster of Excellence and the Collaborative Research Centre 1009 “Breaking Barriers” at the University of Münster.
Original publication:
Wales P, Schuberth CE, Aufschnaiter R, Fels J, García-Aguilar I, Janning A, Dlugos CP, Schäfer-Herte M, Klingner C, Wälte M, Kuhlmann J, Menis E, Hockaday Kang L, Maier KC, Hou W, Russo A, Higgs HN, Pavenstädt H, Vogl T, Roth J, Qualmann B, Kessels MM, Martin DE, Mulder B, Wedlich-Söldner R. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. Elife 2016;5: epub. DOI: 10.7554/eLife.19850. Abstract

