 |
![]()
Name: Florian Hupka
Diploma / M.Sc degree: Westfälische Wilhelms-Universität Münster, Germany
(March 2007)
PhD Project: Self-Assembly of Metallosupramolecular Architectures Based on Benzene-o-dithiol/Catechol Ligands – Trigonal Bipyramidal Clusters and Triple-Stranded Helicates
Abstract of Research Project
Metal-directed self-assembly is widely recognized as an efficient method for the construction of well-defined discrete structures. Besides many fascinating supramolecular complexes that were prepared, one focus of interest is on molecular architectures with a large cavity in the framework. These clusters bear the opportunity to capture short lived intermediates in their interior or to act as “molecular flasks” for catalysis.
Due to the discriminating behavior of the (O-O) and (S-S) donor group, benzene-o-dithiol/catechol ligands have the potential to form large heterobimetallic complexes with suitable transition metal ions. Thereby, one of my PhD projects deals with the assembly of heterobimetallic trigonal bipyramidal clusters, containing three square planar {MIIS4} (MII: d8 metal ions = NiII, PdII, PtII>) and two octahedral {M’O6} (M’ = TiIV, GaIII) coordination polyhedra.
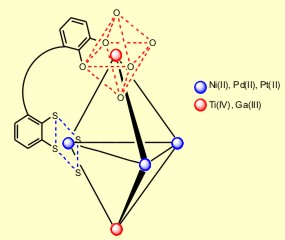
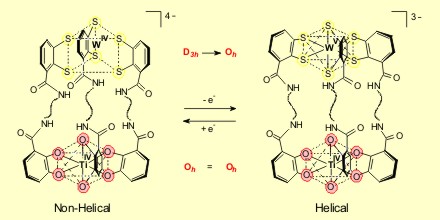
Besides the assembly of supramolecular clusters, another research topic is the synthesis of heterobimetallic triple-stranded helicates. Helicates with oxygen donor functions exclusively contain octahedral coordinated metal centers. With the related tris(benzene-o-dithiolato) polyhedra this is not mandatory. In this case, the metal centers can achieve both, an achiral, trigonal prismatic (d0, d2 metal ion) or a chiral octahedral geometry (d1 metal ion), depending on the formal oxidation state of the metal center.
In particular, I will focus on helicates based on a Ti-W or a Ti-Re metal combination. Due to the redoxvariable coordination geometry at the benzene-o-dithiolato-tungsten unit, it should be possible to change the geometry at this metal center from trigonal-prismatic to octahedral by a simple electron transfer reaction. And if this process is reversible, it should be possible to switch on and off the helicity of these complexes by chemical or electrochemical redox reactions.
Publications
W. W. Seidel, M. J. Meel, S. R. Hughes, R. Stephen, F. Hupka, A. Villinger
Ethenedithione (S=C=C=S): Trapping and Isomerization in a Cobalt Complex
Angew. Chem. Int. Ed. 50 (2011), 12617–12620.
J. Dömer, J. C. Slootweg, F. Hupka, K. Lammertsma, F. E. Hahn
Subcomponent Assembly and Transmetalation of Dunuclear Helicates
Angew. Chem. Int. Ed. 49 (2010), 6430–6433.
F. Hupka, F. E. Hahn
A banana-shaped dinuclear complex with a tris(benzene-o-dithiolato) ligand
Chem. Commun. 46 (2010), 3744–3746.
F. E. Hahn, H. Schröder, T. Pape, F. Hupka
Zinc(II), Copper(II), and Nickel(II) Complexes of Bis(tripodal) Diamide Ligands – Reversible Switching of the Amide Coordination Mode upon Deprotonation
Eur. J. Inorg. Chem. (2010), 909–917.
W. W. Seidel, M. Meel, F. Hupka, J. J. Weigand
Acetylenedithiolate as directional bridging ligand in cobalt(I) alkyne platinum dithiolato bimetallic complexes
Dalton Trans. 39 (2010), 624–631.
M. C. Jahnke, J. Paley, F. Hupka, J. J. Weigand, F. E. Hahn
Silver and Gold Complexes with Benzimidazolin-2-ylidene Ligands
Z. Naturforsch. 64b (2009), 1458–1462.
J. Dömer, F. Hupka, R. Fröhlich, F. E. Hahn
Mono- and Dinuclear Coordination Compounds with Directional Bis(bidentate) Ligands
Eur. J. Inorg. Chem. 24 (2009), 3600-3606.
A. V. Zabula, T. Pape, F. Hupka, A. Hepp, F. E. Hahn
N,S- and N,O-Substituted Stannylenes: Preparation and X-ray Diffraction Studies
Organometallics 28 (2009), 4221-4224.
F. E. Hahn, A. V. Zabula, T. Pape, F. Hupka
Preparation and Molecular Structure of a Cyclic Bisgermylene with two Lutidine Bridging Groups
Z. Anorg. Allg. Chem. 635 (2009), 1341-1344.
M. C. Jahnke, M. Hussain, F. Hupka, T. Pape, S. Ali, F. E. Hahn, K. J. Cavell
Synthesis of pyrazine-bridged diimidazolium salts and their application in palladium catalyzed Heck-type coupling reactions
Tetrahedron 65 (2009), 909–913.
Florian Hupka
eMail: Florian Hupka