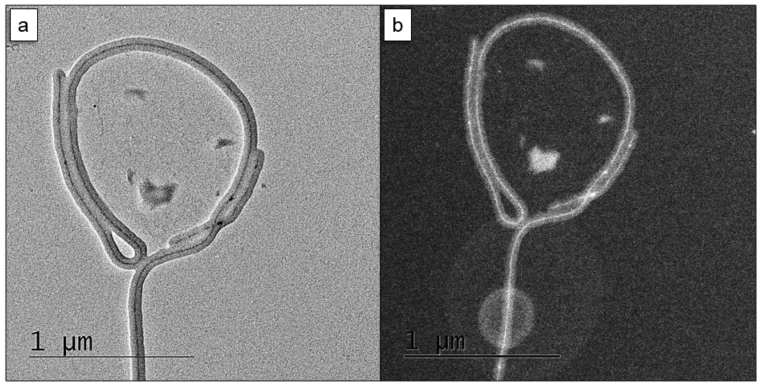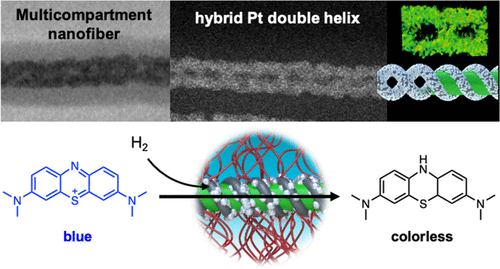
© ACS 2020 Inorganic nanostructures through polymer templates
Block copolymers spontaneously form very defined nanostructures in bulk and solution. The shape and dimension of the compartments can be tuned precisely in the range of 10-100 nm. Just like organic scaffolds direct the formation of complex inorganic skeletons through biomineralization in nature (e.g. bones, silica sponges), we design block copolymer nanostructures as scaffolds to template inorganic materials. The soft nature of block copolymers allows for curved interfaces that direct the growth of inorganic nanostructures with unusual shapes and forms. ABC triblock terpolymers form a rich variety of complex nanostructures identified already in the late 1990s by Reimund Stadler. We now explore their use as geometrically precise scaffolds for mineralization, the formation of multimetallic materials, and as heterogeneous catalysts.
see e.g. Tjaberings S, Heidelmann M, Tjaberings A, Steinhaus A, Franzka S, Walkenfort B, Gröschel AH., ACS Applied Materials & Interfaces 2020, 12, 39586-39594. doi: 10.1021/acsami.0c10385.
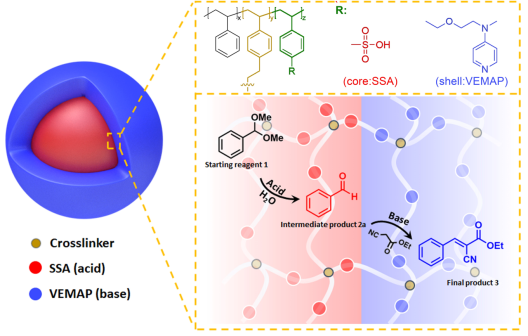
colloidal casade catalyst© A.Gröschel Cascade catalysis in nanoreactors
Compartmentalization is a fundamental concept in nature that allows organisms to separately store, convert and transport matter. Self-assembled compartments allow the site-isolation of catalysts to suppress cross-reactions. The "organs" of cells (organelles) perform catalytic cycles with multiple catalysts that otherwise consume each other. We seek to imitate these biological compartments with polymer compartments to strictly separate “wolf and lamb” catalysts from each other. We from water-soluble multicompartment nanoreactors (MCNR) with specially designed block copolymers and self-assembly concepts or through emulsion polymerization. Cascade catalysis in one-pot nanoreactors in water reduce waste of solvents and energy, and are safer to handle. The nanometer distance between nanocompartments (and catalysts) increases reaction rate and high, and may allow to realize reactions that are inaccessible otherwise, e.g., due to the inactivation of intermediates.
see e.g. Chen C, Janoszka N, Wong CK, Gramse C, Weberskirch R, Gröschel AH, Angew. Chemie Int. Ed. 2020, 60, 237-241. doi: 10.1002/anie.202008104.