RESEARCH - Andrea Rentmeister
CRC1450: Multiscale imaging of organ-specific inflammation
https://www.uni-muenster.de/CRC-inSight/
Our project (with Prof. Mootz):
Cell-specific labeling of immune cells using bioorthogonal chemistry
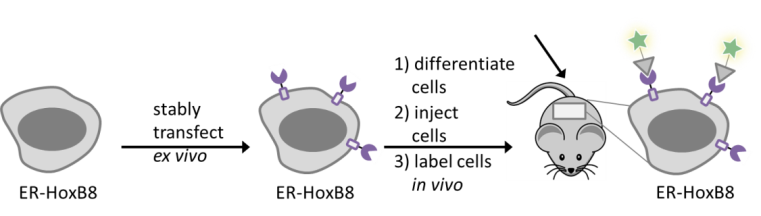
Imaging of different leukocyte populations in vivo is key to understand their dynamics and role in inflammation. To overcome current limitations, we develop methods to label immune cells within a longer time window, i.e., from initiation of inflammation to resolution. Within the framework of the CRC1450, we aim to establish a flexible and modular platform applicable to different imaging modalities, such as fluorescence, scintigraphic or photoacoustic imaging. Cells will be genetically tagged ex vivo with self-labelling enzymes, differentiated and re-injected for in vivo labeling. The latter will be achieved by administering tag-specific, functionalized labels to selectively visualize the respective cell population. Novel approaches will be initially evaluated in a mouse model and then expanded to other disease models in collaboration with other projects from CRC1450.
https://www.uni-muenster.de/CRC-inSight/research/area-A/A01.php
Bioorthogonal chemistry
Chemical labelling of proteins at the cell surface has recently become feasible and is attractive, because small functional groups or labels should not perturb the biological function (Fig. 2). Bioorthogonal functional groups react exclusively with each other under physiological conditions and are orthogonal to the functional groups occurring in biological systems. The reactants and products are non-toxic to living systems and thermodynamically, kinetically as well as metabolically stable under physiological conditions (Sletten 2011). Ideally, such reactions need to operate with fast reaction kinetics in order to label low abundant biomolecules (Fig. 2). Bioorthogonal reactions are modular, meaning that a diverse array of reporters can be installed on biomolecules by a single reaction and that they can be combined with different targeting moieties.
The strain-promoted azide-alkyne cycloaddition (SPAAC) between an azide and a cyclooctyne derivative has been shown to work in cell culture and in animals (Chang 2009). The inverse-electron demand Diels-Alder cycloaddition (iEDDA) of an 1,2,4,5-tetrazine and a strained dienophile (tetrazine ligation, Fig. 2) has recently become the most attractive bioorthogonal reaction for labelling of live cells and in vivo applications (Reiner 2011, Stéen 2019). The kinetics of the reaction can be tuned over 10 orders of magnitude and the dienophile has the most pronounced effect on the reaction rate, with trans-cyclooctene (TCO) being one of the fastest dienophiles applied in biological systems (k2 = 105 M-1s-1) to date (Fig. 2B). The substituents at the tetrazine also affect the rate of the iEDDA reaction. The rapid kinetics of the tetrazine iEDDA cycloaddition makes it an ideal candidate for radionuclide imaging via PET or SPECT, especially for radiolabels with short half-lives (Mayer 2017).
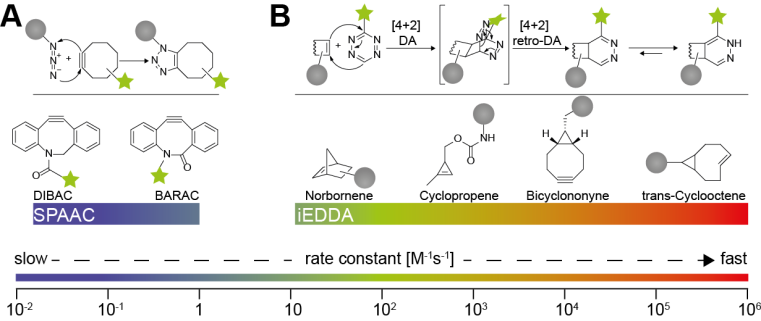
The strain-promoted azide-alkyne cycloaddition (SPAAC) between an azide and a cyclooctyne derivative has been shown to work in cell culture and in animals (Chang 2009). The inverse-electron demand Diels-Alder cycloaddition (iEDDA) of an 1,2,4,5-tetrazine and a strained dienophile (tetrazine ligation, Fig. 2) has recently become the most attractive bioorthogonal reaction for labelling of live cells and in vivo applications (Reiner 2011, Stéen 2019). The kinetics of the reaction can be tuned over 10 orders of magnitude and the dienophile has the most pronounced effect on the reaction rate, with trans-cyclooctene (TCO) being one of the fastest dienophiles applied in biological systems (k2 = 105 M-1s-1) to date (Fig. 2B). The substituents at the tetrazine also affect the rate of the iEDDA reaction. The rapid kinetics of the tetrazine iEDDA cycloaddition makes it an ideal candidate for radionuclide imaging via PET or SPECT, especially for radiolabels with short half-lives (Mayer 2017).