
"DNA would be the thinnest wire in the world"
On 25 April 1953, molecular biologists James Watson and Francis Crick published a paper in which they decoded the double helix structure of the genetic material of all living beings, the DNA. Since 2003 DNA Day has paid tribute to this breakthrough and further ground-breaking work by Maurice Wilkins, Rosalind Franklin and other scientists. Chemist Dr Nils Flothkötter also studied DNA during his doctoral programme in Prof Dr Jens Müller’s working group at the Institute of Inorganic and Analytical Chemistry at the University of Münster - not from a biological point of view, but rather with regard to the question of whether DNA could be used as a component in miniaturised electronic devices in the future. Christina Hoppenbrock spoke with him about this potential innovation on the occasion of DNA Day.

That’s right. The decisive factor lies in its structure: the DNA we used in our research was double-strand DNA. This is normally the case with naturally available DNA, as well. On a molecular level, this structure resembles a kind of wire. If it were possible to use this naturally prefabricated structure as a conductor, it would be the thinnest wire in the world. However, DNA is generally regarded as an insulator. For my doctoral work, I focussed on making DNA accessible for applications in which it can be used as a conductor.
How does it work?
I inserted various metal ions into the DNA, because metals and many metal complexes are able to conduct electrons. To do this, I synthesised artificial nucleobases that can bind silver, copper and mercury ions and incorporated them into the DNA strands as nucleosides, i.e. in combination with a sugar molecule. I focussed on structures that can bind to silver, copper and mercury ions and investigated whether the uptake of these transition metal ions influences electron conductivity.
Indeed, electron conductivity can be significantly increased by incorporating silver ions into the artificial DNA strands. Unfortunately, however, the DNA cannot be considered a conductor even after the transition metal ions have been incorporated.
So is the use of DNA in electronic devices still a dream of the future?
Exactly. In the future, I can best imagine using it in the design and operation of molecular machines or magnets. There are still too many difficulties standing in the way of using them in larger machines in everyday life. For example, the stability of the strands is problematic. After some time, the DNA strands decompose, especially in a liquid medium.
You are now working as a postdoctoral researcher at the MEET Battery Research Centre. Is DNA still an issue for you?
The ‘BIOSTORE’ project, in which I am involved, is about the biologisation of the battery and its components. Unfortunately, DNA research is not far enough along to use these macromolecules in a battery. I could imagine that there would also be major problems with stability. However, in the project we’re still looking at using bio-based materials, such as polysaccharides, and their impact on battery performance. I’d be very happy if I could utilise my knowledge of DNA and incorporate these structures into a battery cell. However, I have my doubts as to whether this will be possible in the near future.
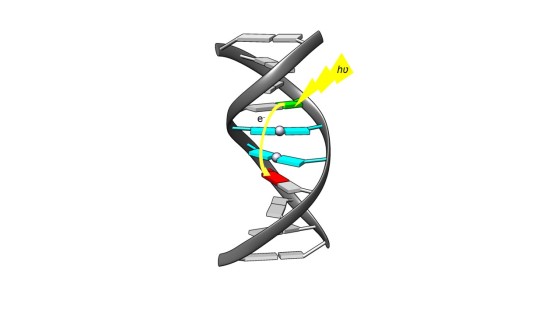
Nils Flothkötter investigated light-induced charge transfer through DNA and evaluated its efficiency. To this end, he produced artificial nucleobases and integrated them into DNA strands. One of these artificial nucleobases releases exactly one electron per strand after being irradiated with light of a certain wavelength. This electron is channelled through the natural and/or metal-mediated base pairs until it reaches a kind of trap. This trap is another artificial nucleobase that decomposes when it receives an electron. Nils Flothkötter calculated the efficiency and a rate constant for the reaction based on the degree of decomposition as a function of the irradiation time.
In the second part of his thesis, he characterised DNA films on gold electrode surfaces using electrochemical techniques. For this purpose, he produced artificial DNA strands that have a so-called “linker” in addition to the metal-mediated base pairs or mispairs. This linker makes it possible to immobilise the double strands on the gold electrode surfaces and then investigate what influence the formation of the metal-mediated base pairs and their position in the double strands have with regard to their electrochemical properties. Nils Flothkötter investigated the influence of the metal ions in the DNA film, whether the electrons are also channelled through the strands in this structure and whether properties such as layer thickness have an influence.
